mieducation
Customisable Diagnostics for Dry Eye: Working with the C.Diag
Dry eye disease (DED) is on the rise, with many of our adult and paediatric patients presenting either asymptomatically, or with symptoms that negatively affect their lifestyle. Having access to diagnostic software to determine the type of dry eye a patient is experiencing, as well as to assist with the best management plan to treat them, is imperative for providing the best care for our patients.
In this article, Dr Jennifer Rayner reviews Lumibird Medical’s C.Diag – a fully automated, comprehensive, and customisable diagnostic tool to diagnose and manage dry eye disease.
WRITERS Dr Jennifer Rayner
LEARNING OBJECTIVES
On completion of this CPD activity, participants should:
1. Realise the prevalence of dry eye disease,
2. Be aware of lifestyle factors contributing to dry eye disease,
3. Understand of the features of C.Diag, and
4. Be aware of recommended steps to treat dry eye.
The global prevalence of DED is increasing at the alarming rate of 5–50%, depending on the population.1 The impact of this on the individual and society is significant, with negative effects on productivity, mental health, and sociability being proportional with increasing DED severity.2 We are even seeing a paediatric population exhibit signs and symptoms of DED, with the prevalence ranging from 5.5%–23.1%.3 This makes it essential for every optometrist to take dry eye disease diagnosis and management seriously.
The TFOS Lifestyle Report Executive Summary, published in 2023,4 stated that lifestyle choices can both directly and indirectly influence the health of our ocular surface, negatively affect the tear film, and contribute to dry eye disease (DED). These include contact lens wear; cosmetics – what we put in and on our skin; interactions with digital devices; the use of topical and systemic medications; periocular and refractive surgery; and influences from the environment, such as humidity, pollution, allergens, and sunlight.
Additionally (which is what I have anecdotally observed in my nine-year practice) the report found factors such as mental health conditions (stress, anxiety, and depression), chronic pain, poor sleep, and nutritional influences – including vitamin deficiencies – are extremely relevant to the incidence of DED. Conversely, omega 3 supplementations and a healthy gut biome have positive roles in maintaining a healthy ocular surface. As well, socioeconomic and cultural conditions can both be contributors.
ASSESSING INDIVIDUAL PATIENTS
As with any medical condition, the more information we have about our dry eye patients – their subtype of DED, risk factors and contributing triggers, and the duration and severity of their presenting symptoms – the better we can individually tailor our treatments for them.
I tell every patient that everyone has their own cause/s for DED, and each person will have different triggers that need to be taken into consideration as part of their optimum treatment plan.
The key to ‘solving’ their dry eye puzzle is obtaining a comprehensive history of when their symptoms started; what their symptoms are; their work history (if retired, what they did before retirement); the medications they are currently taking as well as historical medication (anti-depressants for example); their sleep patterns; stress levels; and diet, for example.
The DEWS II report5 recommends that after asking the patient triaging questions, we do a risk factor analysis, and diagnostic tests to determine the type of dry eye. Tests include a symptom questionnaire and at least one homeostasis marker of either non-invasive tear film break-up time, osmolarity, or ocular surface markers, as a basic dry eye work up.
The diagnosis of evaporative dry eye (EDE) or aqueous deficient dry eye (ADDE) can then be made, and appropriate treatment and management instigated for the individual patient. It is understood that dry eye is now recognised to be on a spectrum of EDE to ADDE, with many subtypes likely on that spectrum. It is worth noting that 86% of DED has some form of EDE.6
Other valuable tests for best management, include looking for blepharitis, Demodex mites, and bulbar redness, as well as performing fluorescein exams for staining.
ADVANCED TECHNOLOGY
With advances in technology, we now have high quality and extensive imaging systems available to us, as well as artificial intelligence (AI), which means we can provide a tailored and often more effective management programme for each of our patients.
Lumibird Medical’s C.Diag platform is one such platform for managing DED. Together with the C.Stim Intense Pulsed Light (IPL) unit, it forms the ‘C.Suite’ – a platform that provides everything needed to professionally diagnose and manage your dry eye patients.
My Experience with the C.Diag
The C.Diag software interface is intuitive. The home screen gives access to the list of entered patients. The unique optical liquid lens camera offers fast and clear images, and the graphics processing unit (GPU) card and computer provides exceptional memory and a high performance in analysis and management of data and results.
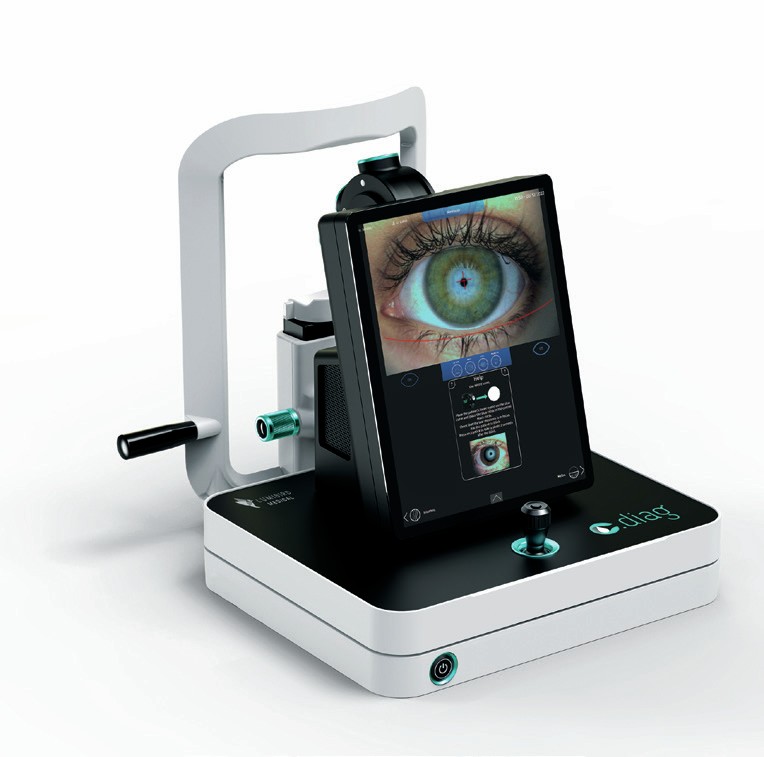
Figure 1. The C.Diag Suite.
It’s easy to find your patient with the search button or add data for new patients. Patient information can also easily be edited or deleted. You can use either the touchscreen or the physical keyboard to enter data and navigate through tests – I prefer the touch screen for ease of use.
All exams can be customised, with for example, automatically suggested comments on the exam performed, adding questionnaires, and suggesting diagnosis. Additionally, users can generate and export a report of the exam session, and ultimately analyse the exams recorded. The technical support settings allow you to create a new user, so if you have multiple practitioners, each can have their own profile. Each user can also choose their own test preference and default protocol sessions if required.
When generating the report, you can select whether to record one or two exams per page. Comments can be made in the patient information summary, such as birthday, email, examination comments (unable to evert upper lids, feels claustrophobia etc.) and any further information about the session. Once the report is completed, it can be downloaded and exported to your desktop, or to a USB, and/or emailed to the patient.
The trolley has good support and is easy to move with automated height adjustment. The chin and forehead rests are fully adjustable and easy to clean. The patient viewing screen makes going through the results with the patient easy.
Selecting Tests
When starting the exam session, clicking on the patient takes you to the default protocols, which include standardised symptom questionnaires (SPEED, DEQ-5, and OSDI); non-invasive keratograph breakup time; blink frequency and amplitude; lipid layer stability; tear film meniscus height; meibography; and valuably, retroillumination of the meibomian glands.
Customisation is easy, as you can select individual tests if you are just repeating meibography, lipid layer analysis, or noninvasive tear film break-up time (NITBUT) on review or after IPL therapy. Additionally, it is simple to add or take out any exams from the default protocol at the time of testing. The default protocol iExam analysis occurs either at the end of the single exam, or when you have finished the whole protocol, depending on what you select. A suggested diagnosis can be included in the report.
Prompts at the beginning of each exam indicate when to change between the white, the Placido discs, and the magnification imaging cones; and there are written tips on how to do each test and patient instructions. This full automation means teaching support staff, learning yourself, and having a uniform protocol to maximise imaging results is easy.
The focus and brightness on each test are easily adjusted at the time, if needed. Testing will default to start on the right – once you’ve done one or both eyes just push the prompts to access the next eye test. You can also go back and repeat any previous test if needed.
Comparing previous exams is easy and the protocols are organised to guide you from less invasive to most invasive testing of the ocular surface to minimise exam interference from subsequent tests.
With the C.Diag using AI, based on 1,200,000 images from over 300 different patient types and collaboration with ocular surface specialists, along with more classical algorithms, the detection, classification and quantification of dry eye disease is readily available at your fingertips. Users benefit from objective evaluations and a clear understanding of the dry eye types. Invaluable images and videos are created to show to the patient and explain the pathology. This helps establish a baseline and provides a visual representation of their improvement as treatments take effect, enabling education on the treatment protocol – this leads to more successful patient outcomes.
CASE STUDY: WORKING THROUGH A DEFAULT PROTOCOL
Deborah Higgin,* is a 56-year-old woman who found our clinic via an internet search. She had been experiencing watering eyes (left worse than right) for the past couple of years, and when mentioning this to her optometrist, she was surprised to find out her reflex tearing was likely due to her underlying dry eye condition. She also complained that her eyes became sore and red when exposed to air conditioning and prolonged screen use. Her diet was unremarkable, but she said she had suffered from eczema and dry skin for most of her life, and had a partial hysterectomy at aged 32 years – she has never taken hormone replacement therapy.
Her past family history included her mother’s diagnosis of scleroderma. She admitted to significant lifestyle stress – she had shingles in 2020, and more recently had another episode affecting her left torso. Her current dry eye treatment of a warm lid compress using a hot flannel and topical artificial tears gave her no relief.
I chose the default protocol of the ocular surface disease index (OSDI) symptom questionnaire, blink frequency and partial blink, tear stability, lipid layer, tear meniscus, and meibography analysis. On initial examination she had moderately inspissated meibum in all glands, and an in-room heat, and lid expression yielded a small amount of expressed meibum. At her second consult, she reported that her symptoms were unchanged.
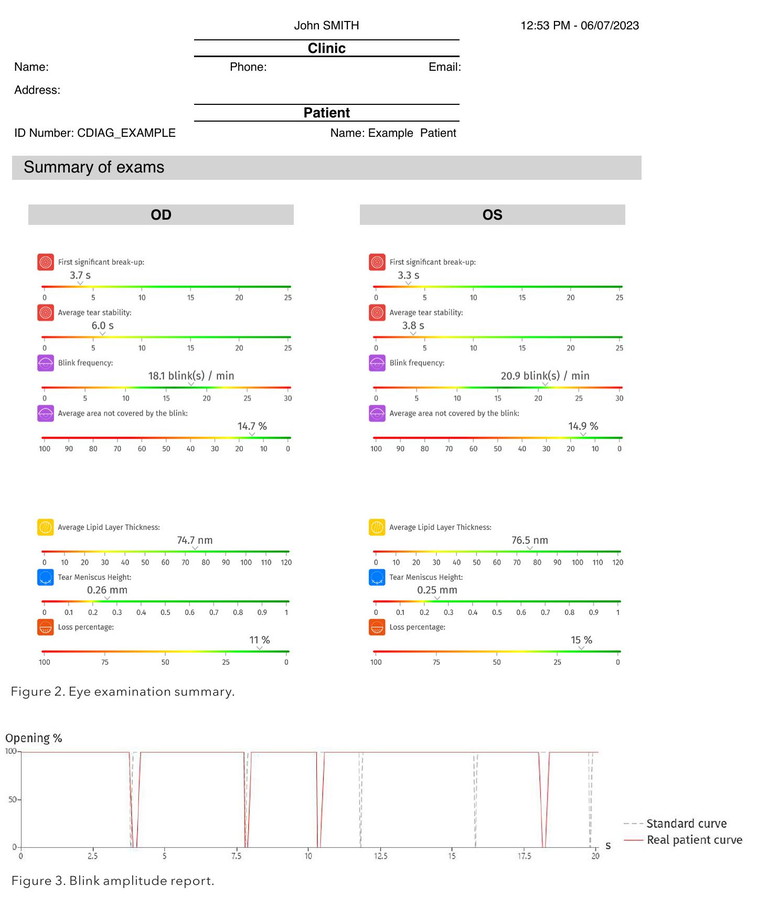
The C.Diag reports give an overall visual summary of the tests. Ms Higgin's blink rate fell within a normal range (from 12 to 18 / minute)7 as seen on the coloured linear grading scale (Figure 2). Blink amplitude and frequency, which are a feature of the blink analysis (Figure 3), are important factors to consider when managing dry eye, as factors such as humidity and excessive screen use can contribute to an unstable tear film,8 which is what she was experiencing. Prolonged ocular surface exposure from decreased blink rates can lead to hyperosmolarity of the eye surface and DED, and incomplete blinking can lead to desiccation of the corneal epithelium.9 As well, meibum is released from the glands on blinking10 and the lacrimal gland producing aqueous is also stimulated by the blink reflex,11 so regular, complete blinks are essential for a stable tear film.
The tear meniscus report also gives a linear scale of low to normal tear film height in millimetres, as well as giving a graphical outline of the meniscus height. Although slightly higher in her left at 0.30 mm than her right (0.15 mm), Ms Higgin did report her watering was not too bad on the day of testing.
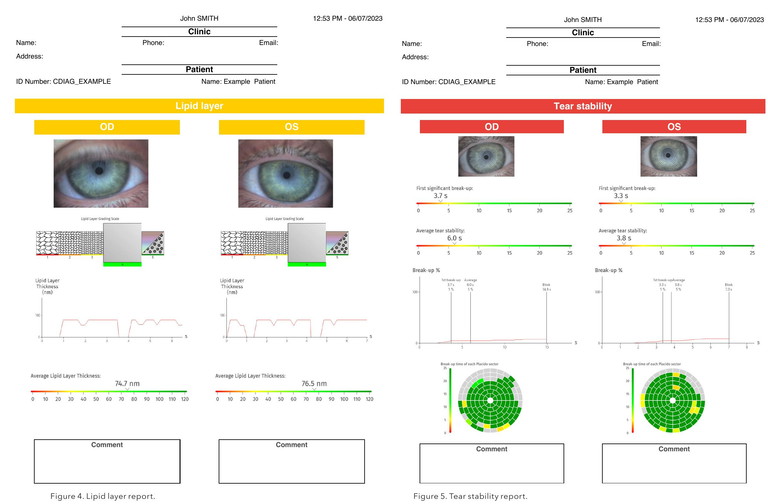
The lipid layer report uses both quantitative analysis (thickness of lipid layer in nanometres) and qualitative analysis to grade severity using the Guillon grading scale.12 Visual imaging of the lipid is available as a video, and comments on severity are provided on the report.
Interestingly, looking at Ms Higgin’s lipid layer analysis (Figure 4), we see her right eye was graded at 4 (not pathological) whereas her left was graded at 1 (severe). Although her tear meniscus height appeared normal, her left was twice that of the height of her right. This could be explained as mild reflex tearing from her poor lipid function.
The tear stability is typically demonstrated by the NITBUT measurement, with the first signs of tear film break-up often occurring in the periphery.
The C.Diag is different as it offers a homogenous projection that is visible to the periphery, giving a precise and complete surface analysis, and using white natural light to accurately reflect reliable, real tear film breakup times. Ms Higgin’s right eye recorded as 2.7 seconds (severe) but her left eye would not capture an image (Figure 5). I have often found that patients with very poor lipid function will not be captured on NITBUT imaging.
When a poor lipid layer is seen, the question is: “are the oil glands simply blocked, or is there little or no function left?”.
The C.Diag offers quantification of the meibomian glands with standard infrared imaging and uses the five-grade scale described by Dr Heiko Pult13 to classify the percentage of gland loss. It also qualifies the gland function using retro-illumination, allowing the user to evaluate the glands’ functionality, meibum production, clogged glands, and telangiectasic vessels. The unique inverter means that meibography imaging is uniform and repeatable, especially when comparing meibomian gland function over time.
“… the more information we have about our dry eye patients… the better we can individually tailor our treatments for them”
Thankfully, Ms Higgin’s lower gland function was grade 1 in the right eye (not pathological), and grade 2 in the left (mild). For the purposes of best resolution for this article, Figure 6 – looking at the retro illumination images – is indicative of her images. It offers good visual of the central ducts and gland function, and mild telangiectasia vessels as expected, as her symptoms had been present for a few years. This would indicate that, at this stage, using IPL therapy for inflammatory management would be appropriate and could also be used to stimulate better meibum production. As well, unblocking her glands using in-rooms expression, or Lipiflow would likely have good effect due to her large percentage of functional oil glands.
At the time of writing this article, she was due to see me for review and a discussion of treatment options based on progress of her signs and symptoms.
Her OSDI gave a total score of 37.5. A score between zero and 12 is considered normal, while a score between 13 and 22 represents a mild disease; between 23 and 32 is moderate DED; and any number between 33 and 100 is indicative of severe DED.14
So why would you need diagnostic software like the C.Diag? Isn’t just observing the tear film with sodium fluorescein and doing a Schirmer test enough?
Alongside triaging questions, risk factor analysis, and diagnostic tests recommended in the DEWS II report to diagnose whether your patient has EDE, ADDE, or any subtype along the spectrum, the C.Diag offers tests for blepharitis, Demodex mites, and bulbar redness, as well as fluorescein exams for staining and tear film break-up time. In analysing each patient, it uses 12 grading scales including the Oxford and Efron, as well as Pult and Guillon, as previously referenced.
YOU NEED TO START SOMEWHERE
With an increasing global prevalence of DED it is essential that as optometrists, we feel comfortable looking for, diagnosing, and treating dry eye effectively.
If you don’t already, start everting the lower lid on every patient to look at their meibomian gland health – you will likely be surprised at how many people asymptomatically have meibomian gland drop out. As well, express the meibum of all your patients. Use a mastrota paddle or dampened cotton bud to apply a counterforce from the palpebral conjunctival area against your finger or another cotton tip to the lid margins of the lower lid (the upper glands are harder to access initially). Again, you may well be astounded at how many people have poor meibum expression. A healthy ocular surface can improve refractive and cataract surgery outcomes as well as support contact lens wear.
For those with signs and symptoms of DED, the 2017 TFOS DEWS II Management and Therapy Report15 recommended treatments that patients can perform themselves, such as warm lid compresses and massage, artificial tear supplementation, and modification of their environment, including, for example, regular screen breaks, blink training and humidifiers.
As practitioners, we have many other tools available to treat DED including IPL, topical steroids, topical ciclosporine, oral tetracyclines, Lipiflow, amniotic membranes, or referral for autologous serum and surgical interventions. Some practitioners will have most or all of them, some will have access to the very basics; but all can be used to manage DED, making your patients much happier and stopping the progressive, inflammatory nature of this disease.
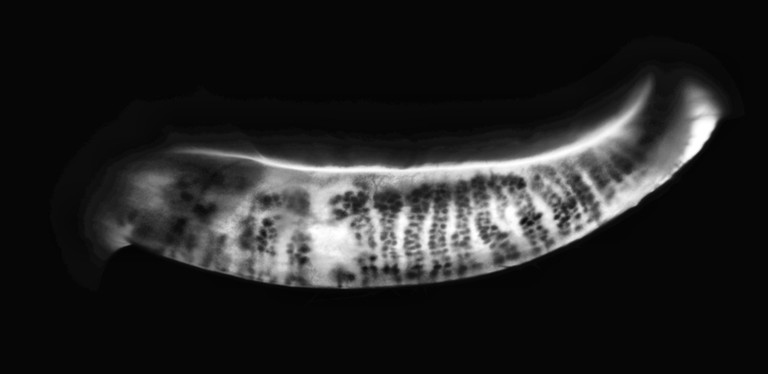
Figure 6. Retro-illumination imaging.
Even if you are not considering the incorporation of an IPL just yet in your practice, at the very least, I cannot recommend highly enough to have diagnostic software such as the C.Diag to help you navigate your way into dry eye management.
Yes – managing DED can initially be frustrating, seemingly illogical, and the cause of a lot of stress, but when you start understanding the pathophysiology of dry eye, along with the risk factors, and you have software to help guide you to the treatment options available, you will be on your way to ongoing successful management.
*Patient name changed for anonymity.

Dr Jennifer Rayner started her career as a registered ophthalmic nurse and assistant before changing careers and graduating as an optometrist in 2003. In 2016 she cofounded South Australia’s first standalone dry eye clinic – Alleve Eye Clinic.
Dr Rayner is passionate about educating both colleagues and the general public on dry eye management. She has presented locally, nationally, and internationally in forums on dry eye management. She sits on advisory boards for emerging pharmaceuticals and treatments, and is a member of the TFOS (Tear Film and Ocular Surface) Lifestyle Workshop public awareness committee.
Dr Rayner is paid by Lumibird Medical to write articles and for speaking engagements.

My dry eye disease website.
To earn your CPD hours from this article, visit mieducation.com/customisable-diagnostics-fordry-eye-working-with-the-cdiag.
This article was sponsored by Lumibird Medical.
References available at mieducation.com.