mieducation
Glaucoma, Imaging, and OCT
Primary open angle glaucoma (POAG), referred to as glaucoma for the rest of the article, is one of the leading causes of irreversible blindness worldwide.
One in 50 Australians are diagnosed with glaucoma, however 50% of people living with the disease are unaware they have it.1 Research has established that glaucoma has a significant impact on a patient’s quality of life2 and an estimated direct annual cost in Australia of AU$144 million.3 Therefore, early detection and accurate diagnosis are essential for slowing the progression of glaucoma to prevent this significant visual loss of the patient and the corresponding financial burden.
When deciding who to screen for glaucoma, there are several known risk factors to consider, such as raised intraocular pressure (IOP) and family history. And while it may seem easier to rely on imaging to help make the final diagnostic decision, as we will see in this article, there are confounding issues that demonstrate why imaging should not be used alone to make a diagnosis.
LEARNING OBJECTIVES
On completion of this CPD article, participants should:
1. Be aware of the evolution of glaucoma imaging,
2. Understand how reference databases can be applied to the analysis of imaging,
3. Realise the limitations of reference databases, and
4. Understand the need to combine imaging, functional testing, and all other risk factors when diagnosing glaucoma.
WRITER Adam Hamilton
Glaucoma is commonly defined as a progressive optic neuropathy characterised by damage to the optic nerve and loss of retinal ganglion cells, resulting in visual field defects.4 This definition helps us to understand why a combination of functional testing and imaging technologies are indispensable in glaucoma management. Optical coherence tomography (OCT) has emerged as a cornerstone of glaucoma diagnostics, enabling clinicians to visualise structural anatomical change at a minute level, as well as detail in the optic nerve head (ONH), retinal nerve fibre layer (RNFL), and ganglion cell complex (GCC) that would be impossible to uncover with any amount of slit lamp observation or fundus photo review. It also creates an easy to understand visual reference for educating patients on both disease progression and results of treatment.
However, the reliance on reference database classification for OCT interpretation introduces challenges that may complicate diagnosis and treatment planning. Are imaging results always right? Can we trust what we see in the images? Is there anything else that the clinician needs to consider when reviewing images, especially in glaucoma?
EVOLUTION OF GLAUCOMA IMAGING
Before the advent of modern imaging, glaucoma diagnosis relied heavily on clinical examination and functional testing. The ophthalmoscope, introduced in the 19th century by Helmholtz, allowed direct visualisation of the ONH. Cup-to-disc ratio assessment became a key parameter for detecting glaucomatous damage, albeit with significant interobserver variability.5
Fundus photography followed, first appearing in the journal Ophthalmology between 1886 and 1888, enabling researchers to document ONH appearance over time.6 It was not until 1926, that the first commercially available unit was brought to market. Stereoscopic fundus photography provided a three-dimensional view, aiding in the assessment of neuroretinal rim thinning (or, more accurately, atrophy) that is characteristic of glaucoma. However, this was a subjective approach and slight changes were not easily detected. With the advent of artificial intelligence (AI), these limitations could be minimised. It will take time for us to know whether AI can enhance our use of fundus photography in glaucoma diagnosis and management.
Functional testing using perimetry, particularly the Goldmann and later Humphrey visual field (VF) tests, became standard for detecting VF defects. These tests remain the standard in defining functional visual loss and we are now seeing virtual reality-based VF testing being introduced by companies such as Olleyes. However, these new methods lack sensitivity in identifying early structural changes preceding functional loss.7
The 20th century witnessed significant advancements in imaging modalities, with the arrival of scanning laser ophthalmoscopy (SLO) technology such as Heidelberg retinal tomography (HRT) from Heidelberg Engineering. The HRT provided 3D tomographic imaging of the ONH, improving assessment of structural changes compared to earlier methods,8 but still lacking highresolution axial imaging and segmentation abilities to differentiate the layers of the retina.9 We also saw scanning laser polarimetry (SLP) devices like the GDx from Laser Diagnostic Technologies (later acquired by Carl Zeiss Meditech), which quantified RNFL thickness by measuring birefringence but was prone to errors in eyes with atypical polarisation profiles.10
We then moved to the OCT, one of the most common imaging tools in modern clinical practice for monitoring disease progression. First introduced in the 1990s, OCT revolutionised glaucoma imaging by providing high-resolution cross-sectional images of the retina.11 Time-domain OCT (TD-OCT) marked the beginning, followed by spectral-domain OCT (SD-OCT), which increased contrast and resolution, and then swept-source OCT (SS-OCT), which enhanced imaging speed. Both SD-OCT and SS-OCT make true monitoring of the tiny changes in glaucoma possible,12 though the latter has reduced axial resolution compared to SD-OCT – a factor that may impact on identification of small changes.
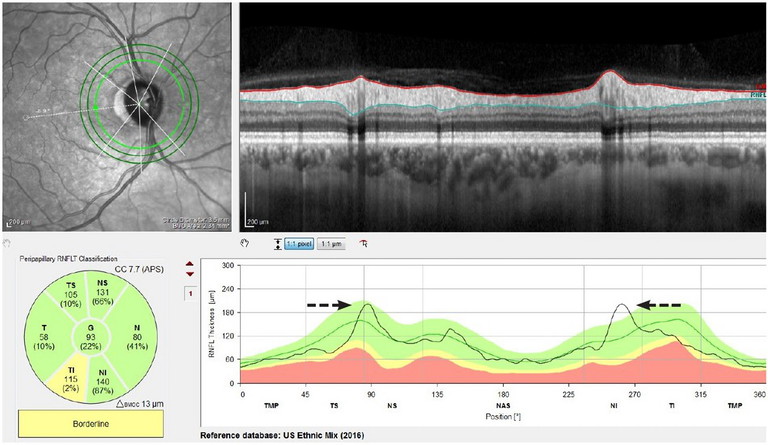
Figure 1. An example of a more vertical orientation of the vessels out of the disc leading to the peaks being pushed inward, causing the temporal inferior segment to flag concern.
“OCT facilitates quantitative analysis of key structures important in diagnosis of glaucoma”
OCT facilitates quantitative analysis of key structures important in diagnosis of glaucoma (however, it is worth noting that different OCT manufacturers denote tissue boundaries differently and measurements may not be interchangeable between devices):
RNFL thickness: Thinning of the RNFL is a hallmark of glaucoma, detectable with OCT even before functional deficits, typically measured at 3.5mm diameter.13
Ganglion cell layer, or various combinations of layers such as the GCC (Topcon Corporation) or GCIPL to ganglion cell-inner plexiform layer (GCIPL) (Carl Zeiss Meditec): Ganglion cell layer analysis of the macular region is particularly useful for differentiating early glaucoma from non-glaucomatous optic neuropathies,14
ONH parameters: Measurements such as rim area/thickness change and cup volume aid in evaluating glaucomatous changes. Some OCT devices employ radial scans around the ONH to assess neuroretinal rim integrity. This approach improves the sensitivity of detecting glaucomatous progression.
ROLE OF REFERENCE DATABASES
A crucial component of the use of OCT technology in glaucoma management is the use of reference databases, which compare an individual’s ocular measurements with a database of presumably healthy individuals.
Reference databases are built by collecting OCT measurements from a large, diverse cohort of healthy individuals of various ages for statistical analysis. Parameters such as RNFL thickness, ganglion cell layer thickness, and ONH are used to quantify and establish normal ranges (through the use of confidence intervals, a range which is expected to typically contain the parameter being estimated, in this case normal, borderline or abnormal measurements).
However, while these databases provide a useful guideline for identifying abnormal findings, they can also introduce errors and biases that may result in either the underor over-diagnosis of glaucoma. Below, we will explore the specific pitfalls that may arise from reliance on reference databases.
Structural Anatomy and Reference Databases
The structural anatomy of ONH and RNFL can vary significantly between individuals. Factors such as optic disc size, shape, and the presence of myopia or hyperopia can alter OCT measurements as described below:
Disc size and shape: Reference databases in some devices assume a standard optic disc size, which could lead to inaccurate interpretations in individuals whose disc anatomy deviates from this norm. A larger disc, for example, might produce an OCT image and subsequent measurement that incorrectly fall outside normative ranges (within the lower 5% of measurements), leading to a false positive.3
Myopia and hyperopia: Individuals with myopia often have thinner RNFLs, which may appear abnormal when compared to a reference database derived from emmetropic individuals. This could result in an overestimation of glaucoma prevalence in myopic populations.15
Vessel arrangement leaving the disc: Retinal blood vessels contribute to RNFL thickness measurements.16 The reference database may not adequately adjust for individual differences in vascular anatomy. This may tend to affect optic disc measurements more so than RNFL thickness, resulting in either an inward shift (peaks in the RNFL scan that are closer together) or outward shift (peaks that are farther apart than the locations on average in the reference database) on the thickness profile, giving the appearance of abnormal areas on what is a normal anatomical variation.
ABNORMALITY AND DIAGNOSTIC SENSITIVITY
Reference databases typically flag abnormalities when measurements fall below a certain threshold, and are often colour-coded as yellow (borderline) or red (abnormal), with the cutoff to these ‘zones’ determined by statistical confidence intervals and standard deviation measures. These thresholds are statistically derived from population data, but they do not always align with the clinical reality of disease progression. Small deviations from the norm may not indicate pathology, as we have just seen, while significant changes may go unnoticed, if they do not cross the predefined thresholds.
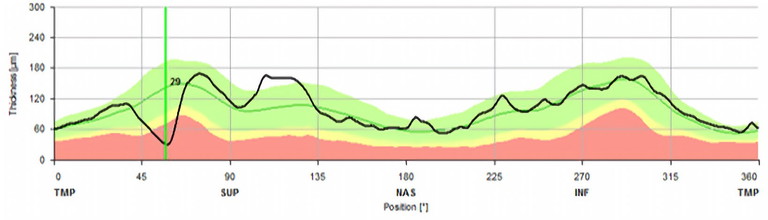
Figure 2. Example of a sharp focal defect. In the early stages of disease, a small focal defect may not show in a sectoral analysis, which is why it is important to look on the B-scan itself.
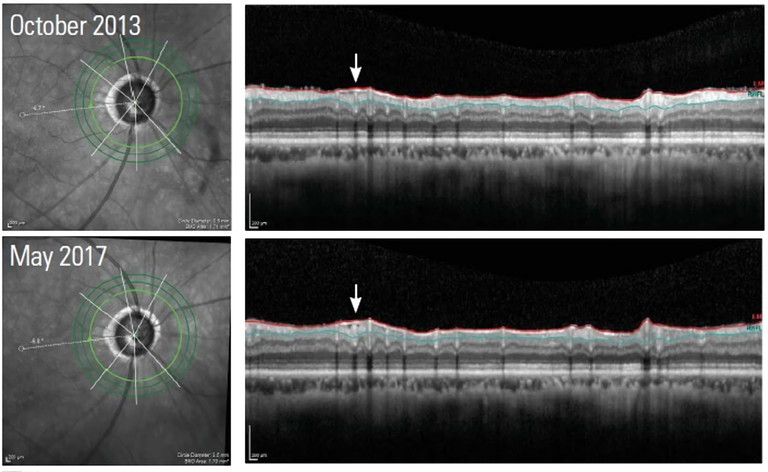
Figure 3. This image illustrates how an epiretinal membrane can cause puckering which ‘hides’ the thinning of the RNFL from detection.
“However, the reliance on reference database classification for OCT interpretation introduces challenges that may complicate diagnosis and treatment planning”
Age is also a significant factor in RNFL thickness (and for that matter many medical diagnoses). In healthy individuals, RNFL thickness naturally decreases with age due to the gradual loss of retinal ganglion cells. Studies have shown that the RNFL thickness decreases at an average rate of 0.5 to 1.0 microns per year in normal ageing eyes,17 whereas glaucomatous eyes are likely to experience loss at a rate of between 1.0 to 2.0 microns per year.13 While reference databases account for age-related decline, interindividual variability remains a challenge. Errors in age-based extrapolation may obscure early glaucomatous changes in older individuals.18
Early-stage glaucoma may produce subtle changes in RNFL thickness that do not cross the thresholds set by reference databases. As a result, glaucoma could go undetected in its early stages, delaying treatment and potentially leading to irreversible vision loss.19 When monitoring glaucoma suspects, it is important to look for sharp focal defects, as these are sometimes masked by sectoral analysis. Furthermore, epiretinal membranes within RNFL scans can also mask these defects due to their tractional nature, though this is more likely to confound monitoring for disease progression.
AI-based systems trained on diverse datasets hold promise for improving diagnostic precision and reducing human related errors.20 AI may be able to augment OCT interpretation by identifying subtle patterns beyond human perception, and may be best used to identify patterns of change across several imaging modalities to support early detection of glaucoma. Additionally, the use of AI in widespread glaucoma screening programmes could potentially lead to earlier disease identification and treatment, reducing the financial burden to patients and the healthcare sector.
Glaucoma progression is often evaluated by comparing serial OCT scans over time. However, reliance on reference databases can complicate this process, particularly if a patient’s measurements fluctuate near the abnormality threshold.17 Minor variations, such as those due to measurement errors and not scanning accurately in the same place, can lead to erroneous diagnosis of glaucoma based on false determination of progression (or lack thereof ). To analyse trends in a glaucoma suspect, it is advisable to look at the temporal superior and inferior zones as well as the global trend plot. The temporal superior and inferior zones can show a steeper slope, indicating tissue loss earlier than will be seen by monitoring the global value alone. This is due to the global value averaging over a greater number of data points, which potentially masks small or local defects.
In advanced stages of glaucoma, disease progression may appear to stop due to the floor effect, where RNFL thickness measurements reach a minimal value beyond which further loss cannot be detected.21 When this limit is reached, OCT of the RNFL (and by extension comparisons to the RNFL normative database) become irrelevant to monitoring progression, and eye care professionals must rely on alternative testing.
THE PROBLEM OF OVER-RELIANCE
OCT and other imaging technologies are only part of the diagnostic puzzle, and when reference databases are used in isolation, we risk overlooking clinical judgment and ancillary test results, which can lead to over-diagnosing, under-diagnosing, and mismanagement of glaucoma. This is especially the case for patients who do not fit neatly into the reference database model.22
Other essential clinical evaluations include IOP measurement, gonioscopy, optic disc examination, and visual field testing. It is also important to take into account other known factors such as family history, ethnicity, age, etc.4
OCT SCANS AND GLAUCOMA MONITORING
To ensure the data derived from OCT images can be trusted, a systematic approach to analysis is essential.
Start with the disc image, which most OCT systems will display in some form. The usefulness of this image for glaucoma diagnosis will vary by device, but it should be in focus and ideally have minimal artifact. This will aid in the assessment of the disc and vessel morphology, and help identify any potential anatomic variations compared to a ‘normal’ disc orientation and the reference database. By seeing how the OCT scans align in relation to the disc, either the technician or the clinician can determine if any change noted is due to inconsistent scan locations across visits.
“Other essential clinical evaluations include IOP measurement, gonioscopy, optic disc examination, and visual field testing”

Adam Hamilton BSc MHRM has 20 years’ experience in the ophthalmic community. Commencing his profession as a new graduate working in an ophthalmic clinic, his passion for imaging was sparked by an old Canon film camera – he would spend days in the dark doing angiograms. Ten years ago he joined Heidelberg Engineering, and currently works as Manager of Professional Education (Asia Pacific), raising awareness about the benefits and potential pitfalls of imaging technology in eye health in clinics around Asia.
Now move onto the OCT scans themselves. Check these scans for motion artifacts that may be caused by patient movement during image acquisition as these can introduce irregularities in the scan, leading to inaccurate measurements.23 While modern OCT devices have faster scan times, motion artifacts remain a concern, especially in systems without live eye tracking (switched on).
Automated layer segmentation should also be checked, as the algorithm may misidentify retinal boundaries, especially in eyes with pathological changes.24 These errors can lead to incorrect RNFL thickness measurements and misinterpretation when compared to reference data. All devices should have this information readily available for review.
Finally, move on to the trend analysis. Check beyond the global value to ensure there is no masking of focal defects. If the RNFL thickness is very thin, check to determine whether the measurement floor been reached.
CONCLUSION
Imaging has transformed glaucoma diagnostics, with OCT serving as a pivotal tool in detecting structural changes preceding functional loss. It is fortunate that this amazing tool (unlike some of those that came before) has a function in detection and monitoring of many other diseases also.
Reference databases have undoubtedly enhanced the detection and management of glaucoma. However, the limitations of these databases must be recognised and carefully considered in clinical practice. Physiological variability and technological limitations can lead to errors in diagnosis and disease monitoring. By combining imaging and functional testing with an understanding of all the other risk factors in glaucoma, clinicians can overcome potential pitfalls and provide more accurate, effective, and timely care for patients with glaucoma.
A systematic approach to glaucoma monitoring and identifying confounding factors are crucial to making the right decision on when to refer, prioritising high quality scans with a holistic assessment of the patient from other clinical exams.
Future advancements in imaging and artificial intelligence hold the potential to further refine diagnostic and therapeutic strategies, ultimately improving outcomes for glaucoma patients.
To earn your CPD hours from this article visit: mieducation.com/glaucoma-imaging-and-oct.
References
1. Glaucoma Australia, What is Glaucoma? Available at glaucoma.org.au/what-is-glaucoma. [accessed Jan 2024].
2. Freeman EE, Muñoz B, West SK, et al. Glaucoma and quality of life: the Salisbury Eye Evaluation.
Ophthalmology 115:233–8. doi: 10.1016/j. ophtha.2007.04.050.
3. Taylor HR, Pezzullo ML, Keeffe JE. The economic impact and cost of visual impairment in Australia. Br J Ophthalmol, 90:272-275. doi: 10.1136/bjo.2005.080986. 4. Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014 May 14;311(18):1901-11. doi: 10.1001/jama.2014.3192.
5. Jonas JB, Dichtl A. Optic disc morphology in myopic primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 1997 Oct;235(10):627-33. doi: 10.1007/ BF00946938.
6. Feibel RM. Looking back at ophthalmic imaging from the first century of the American Journal of Ophthalmology: Photography and ultrasonography. Am J Ophthalmol. 2018 Nov;195:xx-lv. doi: 10.1016/j. ajo.2018.08.020.
7. Quigley HA. Early detection of glaucomatous damage II. Changes in the appearance of the optic disk. Surv Ophthalmol. 1985 Sep-Oct;30(2):111, 117-26. doi: 10.1016/0039-6257(85)90080-3.
8. Burgoyne CF, Crawford Downs J, Bellezza AJ, et al. The optic nerve head as a biomechanical structure: A new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24(1):3973. doi: 10.1016/j.preteyeres.2004.06.001.
9. Lan Y, Henson DB, Kwartz AJ. The correlation between optic nerve head topographic measurements, peripapillary nerve fibre layer thickness, and visual field indices in glaucoma. Br J Ophthalmol. 2003;87:11351141. doi: 10.1136/bjo.87.9.1135.
10. Townsend KA, Wollstein G, Schuman JS. Imaging of the retinal nerve fibre layer for glaucoma. Br J Ophthalmol. 2009 Feb;93(2):139-43. doi: 10.1136/ bjo.2008.145540.
11. Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science, 1991;254(5035):1178-1181. doi: 10.1126/science.1957169.
12. Akil H, Chopra V, Francis BA,et al. Swept-source OCT angiography imaging of the macular capillary network in glaucoma. Br J Ophthalmol. 2017 Aug 9:bjophthalmol-2016-309816. doi: 10.1136/bjophthalmol-2016-309816.
13. Medeiros FA, Zangwill LM, Weinreb RN et al.
Estimating the rate of retinal ganglion cell loss in glaucoma. Am J Ophthalmol. 2012 Nov;154(5):814-824.e1. doi: 10.1016/j.ajo.2012.04.022.
14. Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res, 2007; 26(6):688-710. doi: 10.1016/j.preteyeres.2007.08.001.
15. Biswas S, Lin C, Leung CKS. Evaluation of a myopic normative database for analysis of retinal nerve fiber layer thickness. JAMA Ophthalmol. 2016;134(9):1032-1039.
doi: 10.1001/jamaophthalmol.2016.2343.
16. Hood, DC, Fortune B, Arthur SN, et al. Blood vessel contributions to retinal nerve fiber layer thickness profiles measured with optical coherence tomography. J Glaucoma. 2008 Oct-Nov;17(7):519-28. doi: 10.1097/IJG.0b013e3181629a02.
17. Leung CK, Yu M, Lam DS, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a prospective analysis of age-related loss. Ophthalmology. 2012 Apr;119(4):731-7. doi: 10.1016/j.ophtha.2011.10.010.
18. Harwerth RS, Wheat JL, Rangaswamy NV. Age-related losses of retinal ganglion cells and axons. Invest Ophthalmol Vis Sci, 2008 Oct;49(10):4437-43. doi: 10.1167/iovs.08-1753.
19. Medeiros FA, Zangwill LM, Weinreb RN. Evaluation of progressive neuroretinal rim loss as a surrogate end point for development of visual field loss in glaucoma.Ophthalmology, 2014; 121(1), 100-109. doi: 10.1016/j.ophtha.2013.06.026.
20. Tham YC, Li X, Cheng CY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014 Nov;121(11):2081-90. doi: 10.1016/j.ophtha.2014.05.013.
21. Leung CK, Yu M, Lam DS, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a prospective analysis of age-related loss. Ophthalmology. 2012 Apr;119(4):731-7. doi: 10.1016/j.ophtha.2011.10.010.
22. Wollstein G, Ishikawa H, Schuman, JS, et al. Comparison of three optical coherence tomography scanning areas for detection of glaucomatous damage. Am J Ophthalmol. 2005 Jan;139(1):39-43. doi: 10.1016/j.ajo.2004.08.036.
23. Vizzeri G, Bowd C, Zangwill LM, et al. Effect of improper scan alignment on retinal nerve fiber layer thickness measurements using Stratus optical coherence tomograph. J Glaucoma. 2008 Aug;17(5):341-9. doi: 10.1097/IJG.0b013e31815c3aeb.
24. Sakamoto A, Hangai M, Yoshimura N. Spectral-domain optical coherence tomography with multiple B-scan averaging for enhanced imaging of retinal diseases. Ophthalmology. 2008 Jun;115(6):1071-1078.e7. doi: 10.1016/j.ophtha.2007.09.001.