minews
Green Light for Geographic Atrophy Treatment
“ The approval of Syfovre is a historic moment full of hope for the Australian GA community, who have been waiting for a treatment ”
Syfovre (pegcetacoplan, Apellis) has become the first and only approved treatment for geographic atrophy (GA) in Australia.
The Therapeutic Goods Administration (TGA) approved Syfovre in Australia for the everyother-month treatment of adult patients with GA secondary to age-related macular degeneration (AMD), where the patient has an intact fovea and when central vision is threatened by GA lesion growth.1
The approval of Syfovre was based on results from the Phase 3 OAKS and DERBY studies at 24 months. In the studies, treatment with both every-other-month and monthly Syfovre slowed GA progression and showed a generally well-tolerated safety profile. The results were published in The Lancet in October 2023.2
The only other country in the world to have approved Syfovre to date, to slow down the progression of GA, is the United States. In the United Kingdom, the Medicines and Healthcare products Regulatory Agency decided against approving the drug, expressing concerns over whether it meaningfully improved patients’ ability to see and manage daily tasks.3
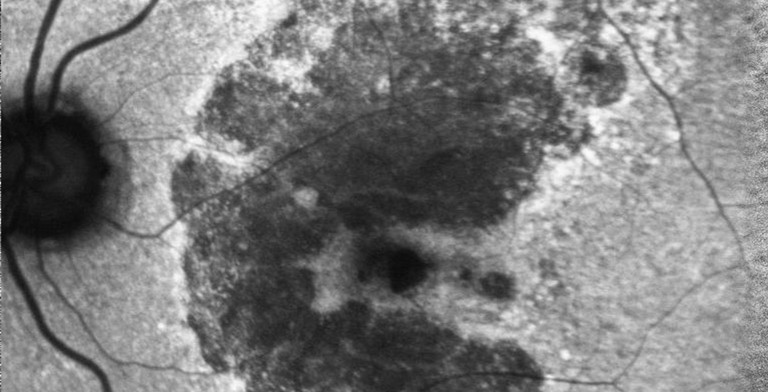
HIGH COST OF GA IN AUSTRALIA
More than 75,000 Australians are living with GA, an advanced form of AMD and a leading cause of blindness worldwide.4 This progressive and irreversible disease is caused by the growth of lesions that destroy the retina’s light-sensing photoreceptor cells, leaving holes with no vision. The vision loss caused by GA severely impairs independence and quality of life.
In late 2024, a report put a price on the cost of GA in Australia for the first time.5 Sponsored by Apellis Australia, an affiliate of Apellis Pharmaceuticals, Geographic atrophy, we can’t wait and see: the case for action, was developed in consultation with Vision 2020, Vision Australia, Retina Australia, Macular Disease Foundation Australia (MDFA), Optometry Australia, and Sight For All.
The report found that GA-related vision loss costs Australia AU$1.8 billion every year. This included $377 million in direct health system costs, $312 million in non-health system costs, and $1.1 billion in lost wellbeing.4
“Much of this cost is borne by people living with GA, who find themselves having to pay high outof-pocket expenses for specialist appointments, eye imaging, and aged care,” the report claimed.
GAME-CHANGING TREATMENT
Retinal specialist and Deputy Director, Centre for Eye Research Australia (CERA) Professor Robyn Guymer AM, was on the global advisory committee for Apellis and led CERA’s site of the international clinical trials into the new drug.
Having “seen how GA often takes away a person’s ability to read, drive, and even see faces of their loved ones”, Prof Guymer said, “the approval of Syfovre is a historic moment full of hope for the Australian GA community, who have been waiting for a treatment”.
While welcoming this “long-awaited positive development for the macular disease community”, MDFA Chief Executive Officer Dr Kathy Chapman also cautioned that despite Syfovre being “independently assessed as being a safe and effective treatment to reduce the impact of geographic atrophy… this outcome doesn’t necessarily mean that they will be eligible for treatment with Syfovre, or that the treatment will be suitable for them”.
Suitability, she said, would be determined by each patient’s ophthalmologist who would “weigh up the benefits and risks” to determine if the new treatment is appropriate.
References available at mivision.com.au.