mieducation
Central Serous Chorioretinopathy: Evolving Understanding, Investigations, and Treatment Options
Central serous chorioretinopathy (CSCR) is an idiopathic retinal disease in which fluid accumulates below the neurosensory retina, causing a serous detachment. CSCR has been typically viewed to be benign due to its self-limiting nature. However, as some cases can be chronic, management can be challenging. Over recent years there have been major evolutions in how we view and manage this classic disease, through deeper pathophysiological understandings and the availability of new treatment options and imaging tools.
WRITERS Dr Long Phan, Jennifer Chin, and Professor Andrew Chang AM
LEARNING OBJECTIVES
On completion of this CPD activity, participants should be able to:
1. Understand the typical signs and symptoms of central serous chorioretinopathy (CSCR),
2. Know the current pathophysiological mechanisms believed to underlie CSCR and the pachychoroid disease spectrum,
3. Be aware of management options, investigational techniques, and treatment indicators for patients presenting with CSCR, and
4. Be aware of masquerade presentations and differential diagnoses associated with CSCR.
Central serous chorioretinopathy (CSCR) is the fourth most common retinopathy, after age-related macular degeneration, diabetic retinopathy, and retinal vein occlusion.1 The defining feature of CSCR is serous retinal detachment, commonly within the macular region, alongside several classical characteristics. Symptoms can include: blurred vision, metamorphopsia, scotoma, micropsia, decreased colour vision, as well as abnormal contrast sensitivity.
Patients may also experience hyperopic shifts in refraction; particularly if there is extensive separation of the central retina.
CSCR typically presents unilaterally at first, however bilateral involvement is not uncommon, especially in older individuals and chronic cases.2 Risk factors for CSCR are well documented and include the male gender, corticosteroid use, autoimmune disease, Helicobacter pylori infection, psychopharmacologic medication use, ‘Type-A’ personalities, smoking, obstructive sleep apnoea, and systemic hypertension.3,4
CASE ONE: CLASSIC ACUTE CSCR PRESENTATION
A 37-year-old male office worker presented complaining of difficulty reading text on the computer screen. He was troubled by work and personal stresses. Visual acuity was 6/6 in the right eye and 6/9 in the left eye, which improved to 6/6 upon pinhole testing. A refraction exam indicated a hyperopic shift in the left eye. An ocular coherence tomography (OCT) scan was performed, which revealed the classic sub-foveal neurosensory detachment in the left eye (Figure 1). The right eye was unremarkable.
OCT is the standard imaging modality to clinically diagnose, manage and follow CSCR. It is a non-invasive and objective way to detect and monitor changes in subretinal fluid (SRF). There was no history of corticosteroid use, smoking or systemic health issues. The patient was counselled on stress reduction and advised to use a +1.00 D monocular reading lens to provide symptomatic relief. At a follow-up visit three months later, there was complete resorption of SRF, and visual acuity returned to 6/6.
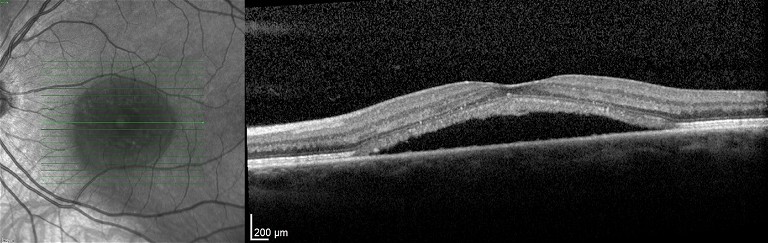
Figure 1. OCT scan of a left eye with CSCR. There is subretinal fluid below the fovea.
Our understanding of the cause of CSCR has evolved over the years. It is currently thought that increased hyperpermeability of the choriocapillaris causes hydrostatic pressure and swelling of the choroid, leading to fluid leakage beneath the retina through defects created in the retinal pigment epithelium (RPE).5
CSCR AS A SPECTRUM OF PACHYCHOROID DISEASE: OCT FINDINGS
CSCR has been recently classified within a spectrum of disorders termed pachychoroid disease.6 The pachychoroid disease spectrum comprises of four disease stages with common characteristics and a shared pathogenesis: pachychoroid pigment epitheliopathy (PPE), central serous chorioretinopathy, pachychoroid neovasculopathy (PNV), and polypoidal choroidal vasculopathy (PCV). All four stages share common characteristics that include: increased choroidal thickening, dilated veins in Haller’s (pachy-veins) layer, as well as thinning in Sattler’s and the choriocapillaris layers.
“ CSCR typically presents unilaterally at first, however bilateral involvement is not uncommon, especially in older individuals and chronic cases ”
In the pachychoroid spectrum, PPE is believed to be the precursor or incomplete form of CSCR where patients show diffuse or focal increases in choroidal thickness with RPE abnormalities such as pigment epithelial detachment (PED).7 SRF may be absent at this stage. Meanwhile, PNV and PCV are considered complications of CSCR, characterised by the development of choroidal neovascularisation (CNV) and PCV respectively.
Enhanced depth imaging (EDI) is a feature of SD-OCT systems that allows visualisation of the choroid with increased clarity. This can help to confirm pachychoroid disease. Choroidal thickness is typically measured from the RPE-Bruch’s membrane complex to the choroidal-scleral interface at the point below the fovea (subfoveal choroidal thickness). However, this may not always be reliable as there is no universal definition for a thickened choroid, due to natural variations in factors such as age, sex, and refractive error. Minimum limits to define a thickened choroid have varied between 300 to 390 μm.8,9
MANAGEMENT OPTIONS FOR CSCR
The goal of treatment is to prevent irreversible vision impairment. This is achieved by eliminating sub-retinal fluid, as persistent fluid can lead to structural photoreceptor damage, retinal atrophy, scar development, and even exudative retinal detachment.10 In severe forms, this can also progress to cystoid retinal degeneration and development of CNV.
Modifying Risk Factors
Risk factor modification can be an effective conservative approach to manage CSCR, particularly in its early stages. Cessation of corticosteroids is a modifiable risk factor, which has been demonstrated to improve CSCR outcomes,11 however this should be done under the management of the treating physician.
Meanwhile, modification of other risk factors – such as smoking cessation, blood pressure control, and stress reduction – have not been proven for efficacy but should be strongly recommended based on overall health benefits.
Photodynamic Therapy
Photodynamic therapy (PDT) involves the intravenous administration of a photosensitive agent, verteporfin (Visudyne, Bausch and Lomb) followed by targeted delivery of low power laser light in areas requiring treatment. The free radicals produced from the excitation of verteporfin via light leads to inflammation, apoptosis, and vaso-occlusion of abnormal choroidal blood vessels. These changes are thought to reduce choroidal hyperpermeability and SRF leakage.12 PDT is currently the treatment of choice for CSCR in the absence of CNV. It is often performed using lower energy laser (half-fluence PDT), to avoid adverse effects such as choroidal hypoperfusion.
Sub-threshold Micropulse Laser
Sub-threshold micropluse laser (SML) involves delivering a series of repetitive ultrashort laser pulses to the retina and RPE. In between each micropulse, there is an ‘off’ period that allows for the tissue to cool down. This method of laser delivery helps reduce the risk of collateral damage and tissue necrosis. The PL ACE study was a randomised clinical trial, which compared SML to PDT in patients with chronic CSCR.13 PDT was found to be superior to SML in terms of fluid resolution, vision gain, and retinal sensitivity. SML may be considered as an alternative laser option to PDT.
Anti-Vascular Endothelial Growth Factor
Intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy conventionally blocks the effects of vascular endothelial growth factor to reduce new vessel growth. However, in CSCR without CNV, anti-VEGF therapy has been applied due to effects on altering vascular and choroidal hyperpermeability. Evidence supporting anti-VEGF therapy in CSCR has been mixed, however recent meta-analysis has confirmed anatomic benefits in patients with chronic CSCR.14
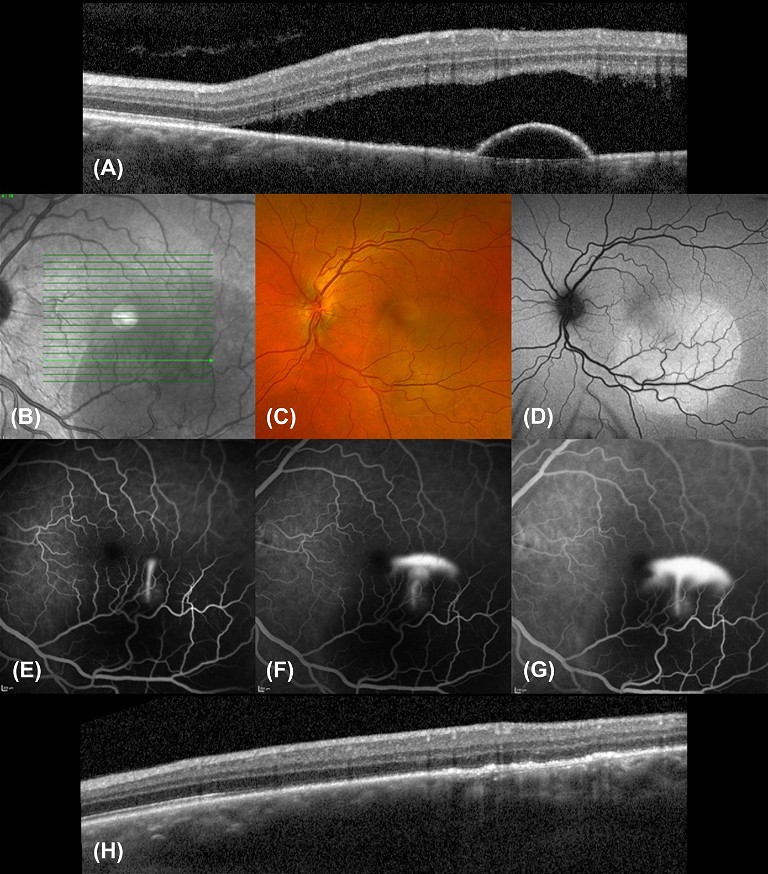
Figure 2. Multimodal imaging of acute CSCR. A) Initial OCT shows SRF with a small serous PED. B) Infra-red (IR) imaging. C) Colour fundus photography. D) Fundus autofluorescence (FAF) imaging shows corresponding area of hyper-autofluorescence representing fluid. E–G) FFA series showing a smokestack leakage pattern. H) Follow-up OCT post thermal laser photocoagulation.
In the clinical setting, when CSCR is complicated by CNV, anti-VEGF has demonstrated both significant visual and anatomic benefits and is considered to be the first line therapy.15,16
Thermal Laser Photocoagulation
Focal photocoagulation using thermal argon laser has been used for decades to directly target areas of leakage in CSCR. This form of laser therapy is typically only reserved for non-central-involving CSCR (>500 µm from the fovea) due to its complications, which can include CNV development (<10% rate) and laser scar development with enlarging atrophy over time, leading to scotomas. While shown to accelerate fluid resorption and visual acuity recovery, it does not appear to significantly reduce the risk of fluid recurrence, and long-term outcomes remain unclear.17
WHEN TO TREAT CSCR
Observation is usually the first step to manage patients with CSCR as treatment is usually reserved for chronic cases (approximately 10–15% of patients).18 However, determining chronicity can sometimes be difficult, as fluid can manifest asymptomatically before presentation to the clinic. The minimum time frame defining chronicity also varies significantly between practitioners, at between three to six months.19 In practice, chronicity alone is not always what determines when treatment is provided. This is because persistent vision loss may occur in patients with acute CSCR after fluid resolution, and 50–60% of patients with acute CSCR can experience fluid recurrence.20-22 Multi-modal imaging of the eye should be used to identify treatment indicators.
CASE TWO: TREATMENT OF ACUTE CSCR
A 43-year-old male presented with progressive blurry vision in his left eye over the past two months (see Figure 2). The OCT revealed sub-retinal fluid overlying a deeper serous pigment epithelial detachment often seen over the point of leak. Sub-foveal PEDs reduce visual prognosis through the development of atrophy,23,24 but have not been linked to chronicity.25,26 While PEDs may not be directly related to serous fluid production, they may indicate the development of an underlying CNV, which may be found in ~10% of cases of CSCR.2
Fundus fluorescein angiography (FFA) was performed to identify the source of leakage and to exclude other diagnoses such as CNV. This patient showed the classic ‘smokestack’ pattern with dye leaking from a point source, which rises to fill the subretinal space. This classic pattern represents focal extrafoveal leakage from the choroidal vasculature. Depending on the location and focality of the leak, thermal laser photocoagulation may be performed if the leak does not resolve spontaneously or to reduce symptoms quickly. The follow-up OCT demonstrated complete resolution of the SRF and PED two weeks after treatment.
CASE THREE: COMPLEXITIES OF CHRONIC CSCR
A 60-year-old male was referred for fluid in his left eye. Presenting visual acuity in the affected eye was 6/12. He reported that a diagnosis of CSCR had been made approximately 20 years ago, and he had laser treatment. OCT scanning revealed a small shallow macular detachment, cystoid macular oedema, and a shallow irregular PED (Figure 3). FAF revealed extensive hyper-autofluorescence and a zone of atrophy. FAF provides a density map of lipofuscin, the predominant ocular fluorophore in the retinal pigment epithelium. Typically, chronic CSCR displays irregular hyper-autofluorescent patterns on FAF. These can be seen as discrete dots, and/or descending gravitational tracts (Figure 4), which represent previous zones of sub-retinal fluid. Angiography with fluorescein and indocyanine green (ICG-A) dye showed multifocal areas of leakage and RPE scarring. The patient was initiated on anti-VEGF therapy. This produced a significant anatomic response, with visual acuity improving to 6/9. He subsequently underwent a kidney transplant with immunosuppressant therapy and returned to the clinic following a dramatic loss of vision to 6/36 in the left eye. OCT confirmed a recurrence of CSCR with a large serous macular detachment, intraretinal cystic fluid, and lipid. This chronic CSCR recurrence was most likely precipitated by systemic corticosteroid use. Anti-VEGF therapy was resumed.
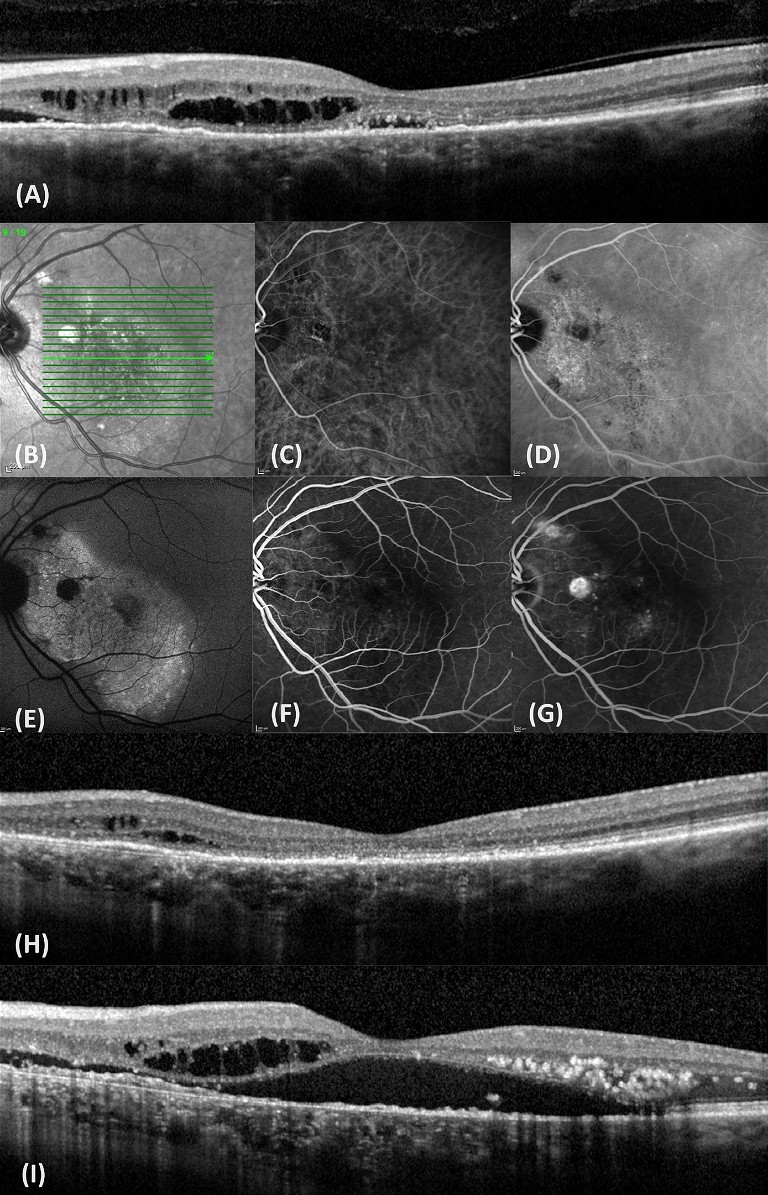
Figure 3. Multimodal imaging of chronic CSCR. A) OCT with intraretinal cysts, SRF, and a shallow irregular PED. B) IR imaging. C&D) ICG-A early and mid-phase revealing hyperfluorescent leakage areas. E) FAF imaging showing extensive hyper-autoflouresence with several discrete dots and a zone of atrophy. F&G) FFA early and mid-phase showing fluid leakage from CNV. H) OCT showing SRF resolution after anti-VEGF therapy. I) OCT after CSCR recurrence.
BEWARE OF MASQUERADE PRESENTATIONS
When a diagnosis of CSCR is suspected, it is important to exclude serious ocular or systemic diseases that may present with serous retinal detachment. Van Dijk and Boon have comprehensively summarised the range of conditions that may manifest in SRF, alongside their differential features.27 Notably, these diseases include: ocular neovascular diseases (e.g. AMD and PCV), vitelliform lesions, inflammatory diseases, ocular tumours, hematological malignancies, medication-related and toxicity-related disease, retinal detachment, and retinal vascular disease. A detailed history and thorough ocular examination with multimodal imaging will confirm these diagnoses.
“ Observation is usually the first step to manage patients with CSCR ”
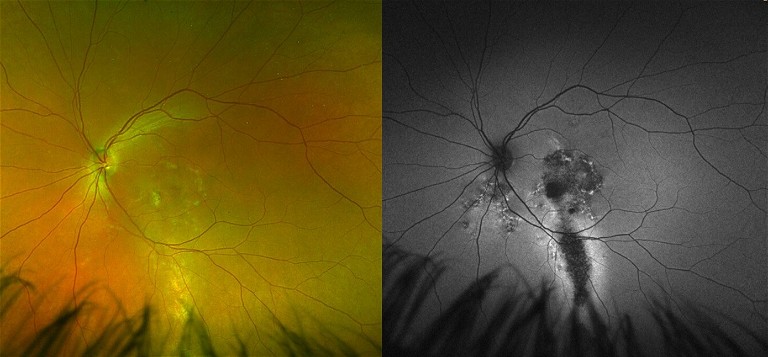
Figure 4. Descending tracts revealed on FAF imaging in a patient with chronic CSCR.
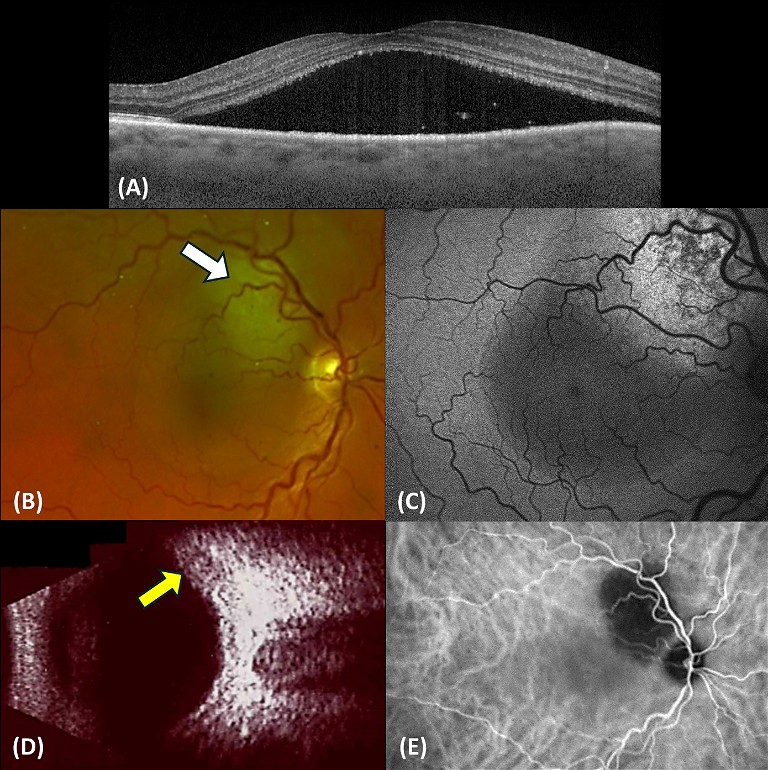
Figure 5: Multimodal imaging of a patient with SRF who was diagnosed with metastatic lung cancer. A) OCT showing SRF as well as some cells. B) Fundus imaging showing a pale solid mass lesion under the retina (white arrow). C) FAF, the acute fluid appears hypo-autoflourescent while the area mass appears largely hyper-autoflourescent. D) B-scan ultrasound imaging, the raised mass can be seen (yellow arrow). E) ICG-A shows a dark zone above the optic nerve where there was blocked choroidal circulation from the lesion.
CASE FOUR: MASQUERADE DUE TO SYSTEMIC MALIGNANCY
A 65-year-old female presented to the clinic after noticing a subacute loss of vision in her right eye over weeks. The patient reported feeling unwell, with lethargy and weight loss. She was a heavy smoker. Visual acuity in the right eye was 6/30. OCT revealed a macular detachment resembling CSCR (Figure 5). On fundoscopy examination, an unusual whitish raised lesion was seen near the superior vascular arcade with undefined margins, which was confirmed using B-scan ultrasonography. This also corresponded to abnormal autofluorescence patterns on FAF imaging. Indocyanine green angiography (ICG-A) was performed to investigate the blood circulation of the choroid.
CSCR alone presents with abnormal dilation of choroidal vessels and multiple areas of choroidal hyperpermeability, particularly in the mid-to-late phases of ICG-A. Meanwhile if there is CNV, early defined lesions are present. For PCV, an abnormal choroidal branching vascular network, followed by hyperfluorescent polyps in the early phase, are diagnostic features. In this case there was a hypo-fluorescent zone corresponding to the blockage of choroidal circulation from the abnormal mass. Unfortunately, the patient was diagnosed as suffering from a choroidal metastasis due to lung cancer.
TAKE HOME MESSAGES
CSCR is a relatively common retinal disease that presents to optometrists and ophthalmologists. Most cases of acute CSCR will settle spontaneously. Chronic and recurrent CSCR may be associated with vision loss due to scarring of the RPE and complications of CNV. Recent understandings of CSCR, as part of the spectrum of pachychoroid disease, has given us clues to pathogenic mechanisms. Multimodal imaging allows the identification of diagnostic features and the consideration of different treatment options, including laser modalities and anti-VEGF therapy. It is important to be aware of and thoroughly investigate masquerade cases of macular detachment, which may indicate serious ocular or systemic pathology.
To earn your CPD hours from this article visit: www.mieducation.com/central-serouschorioretinopathy-evolving-understandingsinvestigations-and-treatment-options.
References
1. Wang, M., Munch, I.C., Larsen, M., et al., Central serous chorioretinopathy. Acta Ophthalmologica. 2008;86(2): 126–145.
2. Spaide, R.F., Campeas, L., Haas, A., et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. Dec 1996;103(12):2070–9; discussion 2079–80. DOI: 10.1016/s0161-6420(96)30386-2.
3. Liu, B., Deng, T., Zhang, J., Risk factors for central serous chorioretinopathy: a systematic review and meta-analysis. Retina. 2016;36(1):9–19.
4. Ersoz, M.G., Arf, S., Hocaoglu, M., Muslubas, I.S., Karacorlu, M., Patient characteristics and risk factors for central serous chorioretinopathy: an analysis of 811 patients. British Journal of Ophthalmology. 2019;103(6):725–729.
5. Donald, J., Gass, M., Pathogenesis of disciform detachment of the neuroepithelium: II. Idiopathic central serous choroidopathy. American Journal of Ophthalmology. 1967;63(3):587/15–615/43.
6. Cheung, C.M.G., Lee, W.K., Freund, K.B., et al., Pachychoroid disease. Eye. 2019;33(1):14–33.
7. Akkaya, S., Spectrum of pachychoroid diseases. International Ophthalmology. 2018;38:2239–2246.
8. Lehmann, M., Bousquet, E., Beydoun, T., Behar-Cohen, F. Pachychoroid: an inherited condition? Retina. 2015;35(1):10–16.
9. Ersoz, M.G., Karacorlu, M., Muslubas, I.S., Pachychoroid pigment epitheliopathy in fellow eyes of patients with unilateral central serous chorioretinopathy. British Journal of Ophthalmology. 2018;102(4):473–478.
10. Wang, M.S., Sander, B., Larsen, M., Retinal atrophy in idiopathic central serous chorioretinopathy. American Journal of Ophthalmology. 2002;133(6):787–793.
11. Sharma, T., Shah, N., Rao, M., et al., Visual outcome after discontinuation of corticosteroids in atypical severe central serous chorioretinopathy. Ophthalmology. 2004;111(9):1708–1714.
12. Erikitola, O., Crosby-Nwaobi, R., Lotery, A., Sivaprasad, S., Photodynamic therapy for central serous chorioretinopathy. Eye. 2014;28(8):944–957.
13. van Dijk, E.H., Fauser, S., Breukink, M.B., et al., Half-dose photodynamic therapy versus high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy: the PLACE trial. Ophthalmology. 2018;125(10):1547–1555.
14. Palakkamanil, M., Munro, M., Sethi, A., Adatia, F., Intravitreal anti-vascular endothelial growth factor for the treatment of chronic central serous retinopathy: a meta-analysis of the literature. BMJ Open Ophthalmology. 2023;8(1):e001310.
15. Chhablani, J., Kozak, I., Pichi, F., et al., Outcomes of treatment of choroidal neovascularization associated with central serous chorioretinopathy with intravitreal antiangiogenic agents. Retina. 2015;35(12):2489–2497.
16. Lai, T.Y., Staurenghi, G., Lanzetta, P., et al., Efficacy and safety of ranibizumab for the treatment of choroidal neovascularization due to uncommon cause: twelve-month results of the MINERVA study. Retina. 2018;38(8):1464– 1477.
17. Ficker, L., Vafidis, G., While, A., Leaver, P., Longterm follow-up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. British Journal of Ophthalmology. 1988;72(11):829–834.
18. Gilbert, C.M., Owens, S.L., Smith, P.D., Fine, S.L., Longterm follow-up of central serous chorioretinopathy. British Journal of Ophthalmology. 1984;68(11):815–820.
19. Singh, S.R., Matet, A., Van Dijk, E.H., et al., Discrepancy in current central serous chorioretinopathy classification. British Journal of Ophthalmology. 2019;103(6):737–742.
20. Nair, U., Ganekal, S., Soman, M., Nair, K., Correlation of spectral domain optical coherence tomography findings in acute central serous chorioretinopathy with visual acuity. Clinical Ophthalmology (Auckland, NZ). 2012;6:1949.
21. Fok, A.C., Chan, .PP., Lam, D.S., Lai, T.Y., Risk factors for recurrence of serous macular detachment in untreated patients with central serous chorioretinopathy. Ophthalmic research. 2011;46(3):160–163.
22. Ozkaya, A., Alkin, Z., Ozveren, M., Yazici, A., Taskapili, M., The time of resolution and the rate of recurrence in acute central serous chorioretinopathy following spontaneous resolution and low-fluence photodynamic therapy: a casecontrol study. Eye. 2016;30(7):1005–1010.
23. Loo, R.H., Scott, I.U., Flynn, Jr H.W., et al., Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002;22(1):19–24.
24. Mudvari, S.S., Goff, M.J., Fu, A.D., et al., The natural history of pigment epithelial detachment associated with central serous chorioretinopathy. Retina. 2007;27(9):1168– 1173.
25. Lai, W-Y., Tseng, C-L., Wu, T-T., Lin, H-S., Sheu, S-J., Correlation between baseline retinal microstructures in spectral-domain optic coherence tomography and need for early intervention in central serous chorioretinopathy. BMJ Open Ophthalmology. 2017;2(1):e000054.
26. Parajuli, A., Joshi, P., Factors influencing the episode duration and the anatomical and functional outcome in cases of acute central serous chorioretinopathy. BMJ Open Ophthalmology. 2020;5(1):e000540.
27. van Dijk, E.H., Boon, C.J., Serous business: delineating the broad spectrum of diseases with subretinal fluid in the macula. Progress in Retinal and Eye Research. 2021;84:100955.

Long Phan BPharm MOrth PhD is a research officer at CUREOS, a clinical research network conducting ophthalmology trials. Dr Phan graduated with a Bachelor of Pharmacy from the University of Sydney in 2016, and a Master of Orthoptics from the University of Technology Sydney in 2018. He subsequently completed a PhD in myopia research, investigating the measurement of risk factors using quantitative measures. He is now involved in retinal research.

Jennifer Chin BVisSci BOptom graduated with a Bachelor of Optometry and Bachelor of Vision Science in 2018. Having practised in both private and corporate settings across regional and metropolitan Sydney, she has encountered a diverse range of ocular pathologies during clinical testing.
Currently working in private practice within Sydney metro, Ms Chin also holds a role as a research liaison officer at CUREOS, fostering connections between clinical practice and research in ophthalmology trials.

Professor Andrew Chang AM MBBS (Hons) PhD FRANZCO FRACS is a vitreoretinal surgeon and ophthalmologist. He holds academic appointments of Conjoint Professor in the Department of Surgery at the University of New South Wales and Clinical Associate Professor at the University of Sydney. He is a consultant ophthalmologist and the Head of Ophthalmology at the Sydney Eye Hospital. He is the Medical Director of Sydney Retina Clinic. Prof Chang was awarded a PhD for retinal research conducted in the Universities of California and Sydney.