mieducation
Evolving Therapies for Geographic Atrophy
Over recent years, investigations into the genetics of age-related macular degeneration (AMD)– and specifically geographic atrophy (GA) – have allowed for deeper understandings of the disease. This article considers the implications of evolving therapies on the treatment of this sight-threatening condition.
WRITERS Dr Alexander Jessup, Dr Long Phan, Jennifer Chin, and Professor Andrew Chang AM
LEARNING OBJECTIVES
On completion of this CPD activity, participants should:
1. Understand the characteristics of GA and its natural history,
2. Be aware of the imaging modalities used to observe geographic atrophy onset and progression,
3. Understand the genetic and systemic risk factors of GA, and
4. Be aware of existing and emerging therapeutic therapies.
Geographic atrophy is an advanced stage of AMD, and the third leading cause of blindness worldwide, affecting 196 million people.1,2 In Australia, 1.3–1.5 million people have AMD and 1–2% of the population over 50 have latestage AMD, half of these with GA.3-5
GA is characterised by increasing cell death (atrophy) in the retinal pigment epithelium (RPE), photoreceptors, and choriocapillaris. These atrophic lesions commonly develop initially in the peri-foveal region and expand towards the macula and fovea, causing progressive and irreversible loss of sight.6,7 Generally, within two years from diagnosis, one third of patients lose three lines or more on a vision chart, two thirds lose their licence to drive, and 42% will be legally and permanently blind.8-10 Visual symptoms worsen substantially when disease progression occurs towards the fovea, leading to central blind spots progressing to total central vision loss. Patients with GA commonly experience difficulty with facial recognition, reading, tasks requiring detailed sight, as well as loss of independence and reduced quality of life.1
Over recent years, investigation of the genetics involved in the disease and adoption of more detailed assessment modalities have allowed for deeper understandings of AMD, and rapid evolutions in the management and treatment of GA. Investigating GA patients on an individual level, even down to their genetic profile, is looking to shape the delivery of treatment in the near future.
RISK FACTORS FOR GEOGRAPHIC ATROPHY
Aside from general risk factors such as older age, diet, and smoking, genetics is heavily involved in both the development and progression of GA. In terms of risk, genome studies have identified several gene variants associated with higher risk of GA. The most consistent and well-known associations involve the age-related maculopathy susceptibility 2 (ARMS2) high-temperature requirement A serine peptidase 1 (HTRA1) genes, as well as genes coding components along the complement system pathway (CFH, C3 and C9).11-15 These major gene loci have also been commonly associated with the rate of GA lesion growth, which also includes a small number of additional genes such as APOE.16,17
The identification of these genetic associations led to the complement system being the initial target for therapeutic developments for GA. The complement system is a part of the innate immune system that evolved to fight infection by controlling inflammation.18 The gene variations associated with GA suggested that abnormal activation of this system was involved in the development and progression of GA. Subsequently, therapies that inhibit various proteins and components along the pathway have been targeted in clinical trials over the years. Given this mode of action, they do not act to reverse GA or improve sight. Although not all have been successful in early phase clinical trials, this pathway remains the main target for current therapeutic development (Figure 1).
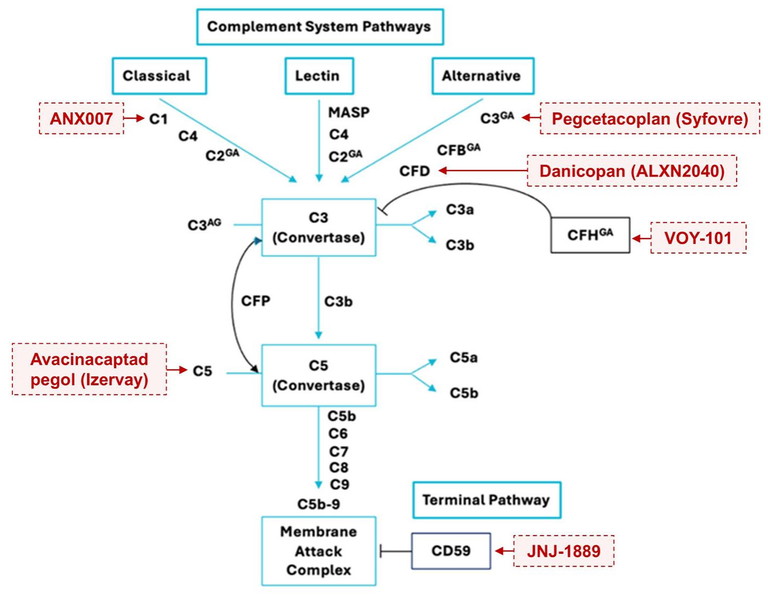
Figure 1. Complement pathways activate a common terminal pathway to form membrane attack complex, leading to RPE damage and atrophy. Complement-inhibiting therapies investigated in clinical trials across Australia are in red. Complement factors with genetic associations to GA are marked with GA. Complement regulators are in black boxes. Diagram adapted from Khandhadia S, et al.37
TREATMENT NOW AVAILABLE IN AUSTRALIA
Treatment and experimental therapies aim to slow the progression of GA and preserve central vision. Until this year, there were no approved treatments available for GA in Australia. On 28 January 2025, the Therapeutic Goods Administration approved pegcetacoplan (Syfovre, Apellis Pharmaceuticals) for GA secondary to AMD. This was the first approved drug for GA, and Australia was the first cohort outside of the United States to be given access to this therapy since US Food and Drug Administration (FDA) approval in 2023.
Syfovre is a solution containing pegcetacoplan, a complement C3 inhibitor given by intravitreal injection into the vitreous cavity of the eye. The OAKS and DERBY studies were two identical Phase 3 randomised clinical trials that investigated pegcetacoplan for efficacy prior to approval. The trials measured GA lesion growth rate to determine drug efficacy. At 12 months, the OAKS trial showed a significant reduction in rate of GA progression by 21% and 16% in groups receiving pegcetacoplan monthly or bi-monthly respectively, compared to a third group receiving a sham injection. In the DERBY trial, similar effects were seen, however the results did not meet statistical significance. However, by the end of the studies at 24 months, both studies showed increased and statistically significant effects of slowing GA lesion progression. In OAKS, pegcetacoplan monthly and pegcetacoplan every other month slowed GA growth rate by 22% and 18% respectively against sham, whereas in DERBY, pegcetacoplan monthly and pegcetacoplan every other month slowed GA growth rate by 19% and 16% respectively against sham,19 with no changes in visual function observed. These studies marked the first time that the growth of GA could be meaningfully slowed by treatment in a large randomised clinical trial.
VARIATIONS IN PATIENT PRESENTATION WITH GA
Pegcetacoplan was approved in Australia to be used once every two months for patients with GA secondary to AMD meeting two characteristics: 1) those with an intact fovea and 2) vision that is threatened by GA lesion growth. High variability in the presentation and prognosis of atrophy can be seen among patients with GA and, given the high treatment burden associated with intravitreal injections known from treating neovascular AMD,20 this specific approval aimed to maximise the benefits of therapy without inducing unnecessary burden onto patients. As practitioners, it is therefore important to determine the suitability of therapy for patients on an individual basis.
GA lesions present in a variety of different ways, which can provide clues to the risk for future progression and potential vision loss. The most vision-threatening features are the size and proximity of the lesion to the fovea. Patients with non-subfoveal GA will likely have better visual acuity than those with foveal involving GA and thus will be at risk of losing sight.21 Larger GA lesions tend to expand at a faster rate than small lesions, while multifocal lesions expand faster when compared to unifocal lesions.22 Aside from the GA, other retinal features can give further clues on the risk of GA progression. The presence of reticular pseudodrusen increases the risk of progression, particularly if located in the macular region.23 Finally, differing patterns of fluorescence surrounding GA lesions on fundus autofluorescence (FAF) images can be examined. FAF uses the property of physiological autofluorescence, when lipofuscin in the RPE is stimulated by light of particular wavelengths to produce a black and white image, where RPE cell death representing GA can be seen as black round areas (hypoautofluorescence). A specific sub-type called ‘diffuse trickling’ FAF pattern has been associated with fastest rates of progression.23
DETECTING, MONITORING, AND ASSESSING GA IN CLINICAL PRACTICE
Approval of the first ever treatment for GA means that front-line eye care professionals will be at the forefront of educating patients and screening for GA treatment. It is important to document and image GA thoroughly to gather information on progression rates, and optometrists are in an ideal position to obtain data, particularly in earlier stages of the disease. This helps not only to contribute to the growth of knowledge surrounding the natural history of the disease, but to also aid in the decision making performed by ophthalmologists on whether or not to treat the GA.
Examining GA features using an appropriate range of multimodal imaging techniques is essential to detect GA, measure and document its progression, as well as assess its risk for vision impairment. The illustrative case shows progression of foveal sparing GA documented over one year (Figure 2). Optical coherence tomography (OCT) is now considered the gold-standard approach to detect and classify GA lesions. The Classification of Atrophy (CAM) consensus group defined GA using the term complete RPE and outer retina atrophy (cRORA), for applications in clinic and clinical trials. This OCT-based definition uses a combination of features, such as signal hypertransmission and the absence of structures in the external retina including the external limiting membrane (ELM), ellipsoid zone (EZ), and RPE.24,25 The advantages of using OCT include the ability to precisely localise the foveal region relative to the GA lesion.
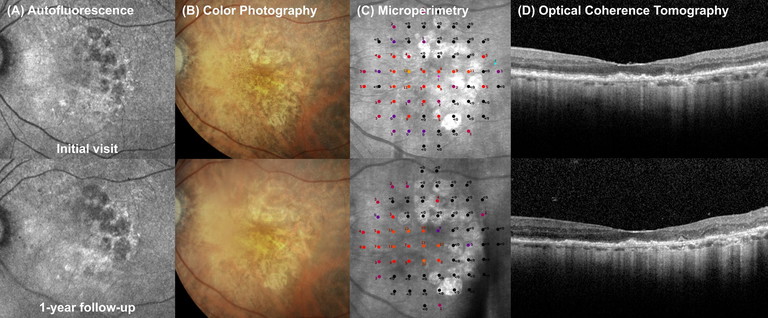
Figure 2. Case of foveal sparing GA progression over one year. An 82-year-old female with foveal-sparing GA in her left eye. VA 6/12, IOP 9. Top row: A) Fundus autofluorescence imaging showed patches of GA with hypo-autofluorescent centres and hyper-autofluorescent borders. B) Colour fundus photography showed hypopigmentation in areas of RPE atrophy. C) Microperimetry showed areas of non-central absolute scotoma (black dots) overlying patches of RPE atrophy. D) OCT showed light hypertransmission through the choroid sparing the fovea.
Bottom row: After one year the patient had documented progression of atrophic lesions, including increased GA size and increased number of scotoma on microperimetry (C). Vision remains stable at 6/9-2. She is now a subject of a gene therapy clinical trial, with the goal of preserving central vision and preventing growth of GA towards the fovea.
To monitor for progression of GA, lesion size needs to be evaluated. Traditionally, GA was captured primarily using fundus cameras. On retinal colour photographs, the size of GA can be measured manually using manufacturer software found on most modern systems, however it can be difficult to precisely determine the location of the fovea. Images from various devices may offer different fields of view, which can potentially distort the appearance and size of lesions due to magnification. It is, therefore, important to maintain consistency when documenting GA over time using this modality. The gold standard method used to measure the size of GA lesions in clinical trials was from blue-wavelength FAF images. FAF imaging is becoming more available in clinics in community practices, with the Heidelberg Spectralis providing the semi-automatic Regionfinder software to measure GA size.
Clinicians should note that there will be some natural hypo-autofluorescence in the fovea due to increased macular pigment, which can make it difficult to assess GA borders close to the fovea.
Near infrared reflectance (NIR) imaging uses longer wavelength light to image the retina. NIR in GA shows a hyper-reflective signal, due to the loss of RPE cells and melanin.25 Compared with FAF, NIR has deeper penetrance of retinal tissue, which can provide more clear delineation of foveasparing lesions.26 Many OCT devices also produce an accompanying NIR image during scanning, which can aid in determining progression rate. Some devices also use NIR to automatically determine GA size, such as the Advanced RPE Analysis on the Cirrus OCT (Carl Zeiss Meditec Inc., USA).
CORRELATION WITH STRUCTURE AND FUNCTION IN GA
To determine the appropriateness of treatment, clinicians now need to correlate structure of GA to its functional impacts when investigating GA. Since vision impairment may not occur until the fovea is significantly involved, additional measures of visual function need to be examined to investigate functional progression and potential threats to visual acuity. Microperimetry measures macular light sensitivity at anatomical points using projected light, a scanning laser ophthalmoscope, and fundus tracking. Microperimetry improves on visual field testing by tracking fixation to provide spatially registered measurements, overcoming problems with fixation. Patients respond to light stimuli projected on the macula at different levels of brightness (measured in decibels), registering their perception by clicking a button. Results show patients’ perimetry grid, colour-coded to represent macular light threshold sensitivity, superimposed on patients’ retinal en face images. Available devices include the Maia (CenterVue), MP-3(Nidek) and OCT-SLO (OPTOS). In GA, areas of atrophy correlate to black spots representing scotoma. With GA progression, the number of scotoma may increase towards the fovea with neighbouring areas showing reduced sensitivity. While not currently commonplace in standard practice, functional measures such as microperimetric testing have been widely used in clinical trial centres and are now becoming an essential requirement for regulatory bodies to assess the effectiveness of upcoming therapies.
CURRENT DIRECTIONS FOR GA THERAPIES
There are more treatment options on the horizon for Australian patients with GA. Avacincaptad pegol (Izervay, Astellas Pharma Inc.) is a complement C5 inhibitor that was approved by the FDA in August 2023,27,28 and is currently under review in Australia by the TGA. Other drug targets for future therapies may inhibit other complement factors, including CFB, CFD, CFH, and CFI.29
Aside from the identification of drug targets, genetics is currently guiding the development of new therapies for GA in several ways. Gene therapy is a promising drug delivery method being investigated for GA patients in clinical trials, which has the potential to permanently slow disease progression from a one-time procedure, rather than injections every two months required by pegcetacoplan.28 The aims of gene therapy for GA require transfer of DNA or RNA genetic material into retinal cells.30 Current ways this has been achieved have been through subretinal, intravitreal or suprachoroidal injections.31 Adeno-associated virus (AAV) is the most common viral vector used to transfer genetic material into the retina.30 The mechanisms of action of introduced genetic material may 1) stimulate gene replacement; 2) inhibit a target gene; 3) alter a target gene; or 4) change phenotype by altering expression of gene networks.30,31
Jaansen Research and Development, the research and development arm of Janssen Pharmaceutical Companies, a subsidiary of Johnson and Johnson, is investigating JNJ1887, delivered through a once-off intravitreal injection.32 It aims to alter retinal cells to stimulate production of CD59, which inhibits the membrane attack complex (MAC) in the complement pathway, preventing RPE cell damage.30 It is currently under trial for efficacy among Australian research sites in a Phase 2b clinical trial (PARASOL, NCT05811351).30,33 The US-based biopharmaceutical company Ocugen’s OCU410 is single intravitreal injection modifier gene therapy targeting multiple pathways in GA.30 The treatment re-expresses the retinoic acid related orphan receptor alpha gene (RORA), regulating ABCA4, CD59, C3, and C5 in the inflammatory pathway.34 OCU410 is being investigated in the Phase 1/2 ArMaDa trial (NCT06018558).34
“The aims of gene therapy for GA require transfer of DNA or RNA genetic material into retinal cells”
Although promising, the efficacy of gene therapy is yet to be known in GA. Potentially, the effectiveness may be related to patients with GA associated with gene dysfunctions. As a result, there has been some more detailed investigation of therapies in a more specific population of GA patients.
Perceive Biotherapeutics, Inc. (also based in the US) is currently investigating VOY-101 another AAV vector-based gene therapy targeting the complement system, in a Phase 1/2a study within Australia (NCT06087458).35 Subjects in this study are required to demonstrate elevated genetic risk for GA through genotyping. If successful, this could mean that in the future, patients will require genetic screening for treatments. However, this could limit the overall benefit of future treatments for the wider population.
Finally, despite modern imaging technologies, it can sometimes be difficult to clinically differentiate GA from dystrophies. Gene classification and identification of GA may be useful to differentiate patients with inherited macular dystrophies.36
CONCLUSION
The approval of the first treatments for geographic atrophy marks a breakthrough in the management of this irreversible sight-threatening disease. The heterogenous nature of the disease drives clinicians towards adopting highly individualised assessment, management, and treatment approaches for each patient, with a combined focus on function and anatomy. Upcoming potential therapies aim to target disease processes using highly innovative drug delivery methods such as gene therapy, and to tailor specific therapies for individuals with genetic risk factors. Ongoing scientific work also aims to further understand earlier stages of the disease and to identify clinically relevant biomarkers to explore preventative measures.
To earn your CPD hours from this activity, visit mieducation.com/evolving-therapies-forgeographic-atrophy.
References
1. Ding L, Kuriyan AE, Sharma G, et al. Weakly-supervised vessel detection in ultra-widefield fundus photography via iterative multi-modal registration and learning. IEEE Transactions on Medical Imaging. 2021;40(10):2748-2758. doi: 10.1109/TMI.2020.3027665.
2. Flaxman SR, Bourne RR, Taylor HR, et al; Vision Loss Expert Group of the Global Burden of Disease Study. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. The Lancet Glob Health. 2017;5(12):e1221-e1234. doi: 10.1016/S2214-109X(17)30393-5.
3. Keel S, Xie J, Dirani M, et al. Prevalence of age-related macular degeneration in Australia: the Australian National Eye Health Survey. JAMA Ophthalmol. 2017;135(11):1242-1249. doi: 10.1001/jamaophthalmol.2017.4182.
4. Fleckenstein M, Schmitz-Valckenberg S, Chakravarthy U. Age-related macular degeneration: A review. JAMA. 2024;331(2):147-157. doi: 10.1001/jama.2023.26074.
5. Macular Disease Foundation Australia. Age-related macular degeneration (webpage). Available at: mdfoundation.com.au/about-macular-disease/age-related-macular-degeneration/amd-overview [accessed March 2025].
6. Bakri SJ, Bektas M, Khan S, et al. Geographic atrophy: Mechanism of disease, pathophysiology, and role of the complement system. J Manag Care Spec Pharma. 2023;29(5-a Suppl):S2-S11. doi: 10.18553/jmcp.2023.29.5-a.s2.
7. Sacconi R, Corbelli E, Querques G, et al. A review of current and future management of geographic atrophy. Ophthalmol Ther. 2017;6(1):69-77. doi: 10.1007/s40123-017-0086-6.
8. Schmitz-Valckenberg S, Nadal J, Fleckenstein M, et al; FAM Study Group. Modeling visual acuity in geographic atrophy secondary to age-related macular degeneration. Ophthalmologica. 2016;235(4):215-224. doi: 10.1159/000445217.
9. Chakravarthy U, Bailey CC, Cantrell RA, et al. Characterizing disease burden and progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125(6):842-849. doi: 10.1016/j.ophtha.2017.11.036.
10. Klein R, Wang Q, Klein B, Moss SE, Meuer SM. The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity. Invest Ophthalmol Vis Sci. 1995;36(1):182-191.
11. Seddon JM, Silver RE, Rosner B. Response to AREDS supplements according to genetic factors: survival analysis approach using the eye as the unit of analysis. Br J Ophthalmol. 2016;100(12):1731-1737. doi: 10.1136/bjophthalmol-2016-308624.
12. Fritsche LG, Igl W, Heid IM, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48(2):134-143. doi: 10.1038/ng.3448.
13. Sobrin L, Ripke S, Seddon JM, et al. Heritability and genome-wide association study to assess genetic differences between advanced age-related macular degeneration subtypes. Ophthalmology. 2012;119(9):1874-1885. doi: 10.1016/j.ophtha.2012.03.014.
14. Yu Y, Reynolds R, Rosner B, Daly MJ, Seddon JM. Prospective assessment of genetic effects on progression to different stages of age-related macular degeneration using multistate Markov models. Invest Ophthalmol Vis Sci. 2012;53(3):1548-1556. doi: 10.1167/iovs.11-8657.
15. Yan Q, Ding Y, Chew EY, et al; AREDS2 Research Group; Weeks DE, Chen W. Genome-wide analysis of disease progression in age-related macular degeneration. Hum Mol Genet. 2018;27(5):929-940. doi: 10.1093/hmg/ddy002.
16. Klein ML, Schultz DW, Acott TS, et al. Age-related macular degeneration: clinical features in a large family and linkage to chromosome 1q. Arch Ophthalmol. 1998;116(8):1082-1088. doi: 10.1001/archopht.116.8.1082.
17. Grassmann F, Fleckenstein M, Weber BH, et al. Clinical and genetic factors associated with progression of geographic atrophy lesions in age-related macular degeneration. PLoS One. 2015;10(5):e0126636. doi: 10.1371/journal.pone.0126636.
18. Janeway C, Travers P, Walport M, Shlomchik MJ. Immunobiology: the immune system in health and disease. Vol 2. Garland Pub. New York, NY, USA; 2001.
19. Heier JS, Lad EM, Wykoff CC, et al. OAKS and DERBY study investigators. Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): two multicentre, randomised, double-masked, sham-controlled, phase 3 trials. Lancet. 2023 Oct 21;402(10411):1434-1448. doi: 10.1016/S0140-6736(23)01520-9.
20. Fleckenstein M, Mitchell P, Ferrara D., et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125(3):369-390. doi: 10.1016/j.ophtha.2017.08.038.
21. Schmitz-Valckenberg S, Sahel J-A, Holz FG, et al. Natural history of geographic atrophy progression secondary to age-related macular degeneration (Geographic Atrophy Progression Study). Ophthalmology. 2016;123(2):361-368. doi: 10.1016/j.ophtha.2015.09.036.
22. Shen LL, Sun M, Del Priore LV, et al. Geographic atrophy growth is strongly related to lesion perimeter: unifying effects of lesion area, number, and circularity on growth. Ophthalmol Retina. 2021;5(9):868-878. doi: 10.1016/j.oret.2020.12.002.
23. Holz FG, Bindewald-Wittich A, Schmitz-Valckenberg S, et al; FAM-Study Group. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol. 2007;143(3):463-472. doi: 10.1016/j.ajo.2006.11.041.
24. Sadda SR, Guymer R, Staurenghi G, et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT: classification of atrophy report 3. Ophthalmology. 2018;125(4):537-548. doi: 10.1016/j.ophtha.2017.09.028. Erratum in: Ophthalmology. 2019 Jan;126(1):177. doi: 10.1016/j.ophtha.2018.10.038.
25. Souied E, Semoun, O, Capuano, V Capturing geographic atrophy with multimodal imaging: Noninvasive technologies are redefining how we diagnose and monitor atrophic AMD. Retina Today. Available at: retinatoday.com/articles/2024-apr/capturing-geographic-atrophy-with-multimodal-imaging [accessed Feb 2025].
26. Querques G, Souied EH. Combined angiography for high-quality near-infrared autofluorescence images. Optom Vis Sci. 2014;91(1):e9-e13. doi: 10.1097/OPX.0000000000000110.
27. Shakeel L, Khan A, Akilimali A. Izervay (avacincaptad pegol): paving the way for vision preservation in geographic atrophy. Ann Med Surg (Lond). 2024;86(5):2413-2416. doi: 10.1097/MS9.0000000000002021.
28. de Oliveira Figueiredo EC, Bucolo C, Eandi CM. Therapeutic innovations for geographic atrophy: A promising horizon. Curr Opin Pharmacol. 2024;78:102484. doi: 10.1016/j.coph.2024.102484.
29. Desai D, Dugel PU. Complement cascade inhibition in geographic atrophy: a review. Eye (Lond). 2022;36(2):294-302. doi: 10.1038/s41433-021-01765-x.
30. Jamil MU, Waheed NK. Gene therapy for geographic atrophy in age-related macular degeneration: current insights. Eye (Lond). 2025;39(2):274-283. doi: 10.1038/s41433-024-03463-w..
31. Drag S, Dotiwala F, Upadhyay AK. Gene therapy for retinal degenerative diseases: progress, challenges, and future directions. Invest Ophthal Vis Sci. 2023;64(7):39. doi: 10.1167/iovs.64.7.39.
32. Lad EM, Chao DL, Heier JS, et al. Pooled safety analysis of a single intravitreal injection of JNJ-1887 (gene therapy, AAVCAGsCD59) in patients with age-related macular degeneration (AMD). Invest Ophthal Vis Sci. 2023;64(8):732-732.
33. A Study to Evaluate Intravitreal JNJ-81201887 (AAVCAGsCD59) Compared to Sham Procedure for the Treatment of Geographic Atrophy (GA) Secondary to Age-related Macular Degeneration (AMD) (National Library of Medicine) (2023).
34. Akula M, McNamee S, Haider NB., et al. Retinoic acid related orphan receptor α is a genetic modifier that rescues retinal degeneration in a mouse model of Stargardt disease and dry AMD. Gene Ther. 2024;31(7-8):413-421. doi: 10.1038/s41434-024-00455-z.
35. Study of VOY-101 in Patients With Advanced Non-Neovascular Age-Related Macular Degeneration (National Library of Medicine) (2024).
36. Gelman R, Tsang, SG. Masqueraders of Age-related Macular Degeneration: A number of inherited retinal diseases phenocopy AMD. Retina Today. Available at: retinatoday.com/articles/2011-apr/masqueraders-of-age-related-macular-degeneration [accessed Feb 2025].
37. Khandhadia S, Cipriani V, Yates J, Lotery AJ. Age-related macular degeneration and the complement system. Immunobiology. 2012;217(2):127-146. doi: 10.1016/j.imbio.2011.07.019.

Dr Alexander Jessup MD MS (Res) BMedHlthSc BA is an ophthalmology researcher at Sydney Retina. He has worked on clinical and surgical research. Current research areas include artificial intelligence, multimodal retinal imaging, and macular diseases.

Dr Long Phan BPharm MOrth PhD is an orthoptist and researcher. He conducts investigatorinitiated research at CUREOS, a clinical research network conducting ophthalmology trials in retinal disease. He has also been involved in several pharmaceutical sponsored trials as a coordinator and examiner. He teaches in the Master of Orthoptics program at the University of Technology Sydney.

Ms Jennifer Chin BVisSci BOptom is an optometrist and research liaison officer. Having practised in both private and corporate settings across regional and metropolitan Sydney since 2018, she has encountered a diverse range of ocular pathologies during clinical testing. At CUREOS, she aids in fostering connections between clinical practice and research in ophthalmology trials.

Professor Andrew Chang AM MBBS (Hons) PhD FRANZCO FRACS is a vitreoretinal surgeon and ophthalmologist. He holds academic appointments of Conjoint Professor Department of Surgery UNSW and Clinical Associate Professor at the University of Sydney. He is a consultant ophthalmologist and the Head of Ophthalmology at the Sydney Eye Hospital. He is the Medical Director of Sydney Retina Clinic. Prof Chang is clinician advisor to the Department of Health Australia and to the Therapeutic Goods Administration on new technologies in ophthalmology. In 2023 his contributions to ophthalmology and retinal surgery were acknowledged in his being awarded as a Member of the Order of Australia (AM).