mieducation
A Paradigm Shift in Refractive Surgery: Wavelight Plus Ray Tracing LASIK
Corneal-based refractive surgery is currently undergoing a transformation with the introduction of the first commercially available ray tracing-based procedure. Ray tracing represents a significant advancement over traditional techniques by offering a new level of personalisation for correcting myopia and myopic astigmatism, resulting in very high early visual results. Ray tracing represents a generational upgrade in laser eye surgery treatment and this article explores the background and progress that has led to the first ray tracing system being launched in Australia this year – the InnovEyes Sightmap device and Wavelight Plus treatment (Alcon, USA).
WRITER Dr David Gunn
LEARNING OBJECTIVES
On completion of this CPD activity, participants should be able to:
1. Understand the evolution of corneal based refractive surgery,
2. Realise the different procedures currently used for laser vision correction,
3. Understand the benefits of customisation for particular patients, and
4. Realise the potential outcomes achievable by applying ray tracing technology to refractive surgery.
LASER EYE SURGERY BACKGROUND
Laser refractive surgery works by changing the shape of the cornea to treat myopia, astigmatism, hyperopia, and presbyopia.
This includes the three main procedures of photorefractive keratectomy (PRK), laser in-situ keratomileusis (LASIK), and laserassisted lenticule extraction (LALEX).
Photorefractive Keratectomy
Surface ablation procedures, such as PRK and transepithelial PRK (trans-PRK ), use an excimer laser to remove anterior corneal stromal tissue. Each spot of laser that treats the cornea removes or ‘ablates’ an exact amount of stroma and very accurately reshapes the cornea. PRK is highly effective but, due to the epithelial defect left after treatment, there is discomfort and an extended visual recovery time after surgery.
Laser In-Situ Keratomileusis
LASIK was described in 1990 using a microkeratome (a small vibrating blade) to create a flap, followed by excimer laser treatment under the flap.1 The creation of this flap led to faster visual recovery and a more comfortable postoperative experience – LASIK quickly became the most popular laser vision correction procedure worldwide.2 LASIK has been performed for over three decades, and improvements in diagnostic and laser platform technology have led to progressively improved visual outcomes, patient satisfaction, as well as significantly lowered risks.2 These days, most LASIK flaps are created by femtosecond (FS) lasers rather than a microkeratome blade. This laser works by making a layer of closely spaced bubbles in the cornea (a process called photo disruption) that is then divided with a blunt instrument. This achieves better precision, faster visual recovery, and a lower risk of flaprelated complications than when using a mechanical blade.3

The Sightmap device.
Laser-Assisted Lenticule Extraction
LALEX has often been termed ‘third generation’ laser vision correction. Here there is no use of the excimer type laser. Instead, the femtosecond laser creates two interfaces in the cornea that form a ‘lenticule’ that is then removed through a small incision leading to a refractive change. In 2007, the Visumax FS laser (Carl Zeiss Meditec, Germany) was launched along with the first refractive lenticule extraction procedure called ReLex.4
“ LASIK has been performed for over three decades, and improvements in diagnostic and laser platform technology have led to progressively improved visual outcomes ”
The Queensland Eye Institute team performed its first Wavelight Plus procedure in April this year.
Dr David Gunn demonstrating the Sightmap device used in Wavelight Plus procedures.
Since then, a variety of companies have introduced their own lenticule procedures, each with their own trademarked brand name – SMILE (small incision lenticule extraction) and FLEX (femtosecond lenticule extraction) both from Carl Zeiss; CLEAR (corneal lenticule extraction for advanced refractive correction, Ziemer); SILK (small incision lenticule keratomileusis, Johnson and Johnson Vision); and SmartSight (Schwind). In 2023, due to the proliferation of these confusing terms and brand names, the Refractive Surgery Alliance agreed upon ‘laser-assisted lenticule extraction’ (LALEX) as the preferred umbrella term for lenticule based procedures.5
Patient work-up using the Sightmap device.
INTRODUCTION OF CUSTOMISATION
Refractive surgery has undergone remarkable advancements since its inception, evolving through several generations of technology, each aimed at improving outcomes and patient satisfaction. Basic ablation procedures initially treated the cornea as a simple thin lens.6 These procedures applied a uniform treatment to the corneal surface based on the spherical and cylindrical prescriptions measured during a standard eye examination. The aim was to modify the corneal power to change the eye’s overall refractive power to achieve emmetropia, where light rays focus directly on the retina for clear vision.
These simple ablations relied on a one-sizefits-all approach that did not account for individual variations in corneal anatomy or optical characteristics. While effective for many, this approach could lead to suboptimal outcomes for patients with unique corneal properties or those with higher prescriptions.
LIMITATIONS OF BASIC ABLATIONS
A limitation of this approach was its reliance on the paraxial approximation – a simplification that assumes light rays travel close to the optical axis and at small angles. While suitable for basic vision corrections, this approximation does not account for how real eyes behave, especially in peripheral areas where light rays enter the eye at wider angles.
This simplification led to the induction of higher order aberrations, particularly spherical aberration where light rays do not converge at a single point, resulting in a blurrier image, especially under low light conditions.7,8
Additionally, early laser treatments did not take into account the ‘cosine effect’. This issue arises from laser pulses having relatively less effect when directed into the periphery of the cornea than in the centre, due to the angle at which they contact the tissue.
EVOLUTION TO WAVEFRONT-OPTIMISED TREATMENTS
Wavefront-optimised (WFO) treatment refers to the wavefront of the laser light. It recognises the cosine effect and provides relatively more laser pulses to the periphery of the cornea to counteract this effect. The intended effect of this was to control spherical aberration induction, leading to improved vision at night. While WFO ablations aim to reduce treatment-induced spherical aberrations, they do not actively reduce the underlying higher order aberrations.8
EARLY CUSTOMISATION – WAVEFRONT-GUIDED TREATMENTS
Topographic irregularity is seen in 10–40% of ‘normal’ corneas that have not been diagnosed with ectatic disease such as keratoconus.9 Conventional excimer treatment profiles may lead to deterioration in postoperative visual quality, night vision issues, and an increase in irregularities, especially in asymmetric corneas.
Attempts to deal with these irregularities became mainstream with the introduction of wavefront guided (WFG) techniques that involved using an additional diagnostic device (such as a Hartman-Shack aberrometer) to collect whole eye wavefront data. This data included information specific to the eye rather than just the lower order aberrations of sphere and cylinder. Higher order aberrations such as coma, trefoil, and spherical aberration, could additionally be measured and treated.
The results in normal healthy eyes were similar or perhaps slightly inferior to the simpler wavefront optimised treatment.10 In eyes with poorer corrected vision beforehand, or with higher aberrations before surgery, WFG treatment could lead to visual improvements over conventional treatments. Generally, it was recommended only to be used when preoperative aberrations were increased and is not commonly performed recently.
TOPOGRAPHY-GUIDED TREATMENTS
A recognition of the anterior corneal surface as the main refractive element of the eye and the main source of aberrations led to the development of topographic guided (Topo-G) treatments. Topography-guided LASIK uses detailed maps of the cornea’s surface to guide the laser ablation process. This technology focusses on the cornea’s curvature and irregularities, providing highly customised ablations that account for minute variations in corneal shape. Topo-G treatments are particularly beneficial for patients with irregular astigmatism, or previous corneal surgery, where the corneal surface may not be uniformly smooth.
Alcon released its Topo-G treatment on the Wavelight excimer laser under the brand name Contoura and its United States Food and Drug Administration (FDA) trial showed some of the best visual results ever reported for LASIK.11 One in three patients had better uncorrected vision after surgery than they had before corrected in glasses or contact lenses. This was a tightly controlled study where patients had minimal topographic irregularity and very high agreement between the astigmatism measured subjectively (in their glasses prescription) and objectively (on the corneal topography). Here it was shown the promise of what true customisation could achieve – making the good better.
CHALLENGES WITH TOPOGRAPHY GUIDANCE
When Contoura was released, performances in real world settings were disappointing, with refractive surprises not uncommon. This was due to an important concept in optics – the inherent linkage between higher and lower order aberrations. Change one aberration in isolation and it will cause a shift in others – for example reducing coma in an eye will lead to a change in the magnitude and axis of the astigmatism. Change the spherical aberration and it will affect the spherical component (myopic or hyperopic shift). In other words, if a significant amount of higher order aberration is treated on the cornea, there will be a significant shift on the resulting sphere and cylinder, potentially leading to a refractive surprise. Some surgeons stopped performing Topo-G treatments due to these issues, considering that WFO gave such great results. For those that persisted, the question with Topo-G treatments was this: How much should I modify the sphere and cylinder of the final treatment, considering what I see on the cornea?
MAKING TOPOGRAPHY-GUIDED TREATMENTS WORK
A variety of methods were created to try to account for this problem by modifying the treatment astigmatism and sphere based on the measured corneal astigmatism such as LYRA (layer yoked reduction of astigmatism protocol) and TMR (topography modified refraction). Both methods showed improvement in results compared to treating the manifest refraction.12,13 The Phorcides Analytic Engine software package was released that calculated a final treatment plan by analysing the higher order aberration treatment map, the anterior and posterior corneal curvature, manifest refraction, and nomogram adjustment. It showed improved results for guiding Topo-G treatments over the manifest and TMR method.14 A prospective multicentre Phorcides trial showed, at three months post-operatively, 100%, 89%, and 28% of patients achieved uncorrected distance visual acuity (UDVA) of 6/6, 6/5, 6/4.5 or better, respectively. Postoperative corrected distance visual acuity (CDVA) was better than preoperative CDVA by one line in 40% and two lines in 6%.15 Topo-G treatments have now reached mainstream recognition and these tools seem to give excellent, predictable results that are superior to previous methods.
WHAT ABOUT LALEX AND SMILE?
As discussed, femtosecond lasers work by ‘photo disruption’, which creates small bubbles in the cornea. Excimer lasers work by ‘photo ablation’ where tissue is removed from the surface by direct light energy that breaks molecular bonds, releasing tissue into a gas plume.
Due to this difference in mechanism, photo ablation from an excimer laser gives submicron precision, allowing extremely fine control of tissue removal at a level not currently possible with femtosecond lasers.16 As such, despite 17 years since the introduction of femtosecond LALEX procedures, complex treatments such as wavefront-guided, topographic-guided, presbyopic, and now ray tracing procedures have been unavailable with that procedure class.
Topo-G and SMILE results, compared in a contralateral eye study in 2017, showed significantly superior results for Topo-G over SMILE in terms of UDVA, percentage of eyes that gained CDVA, and remaining refractive error, as well as subjective patient measures at three months post-procedure.17 Another comparison study found better early postoperative UDVA and better contrast sensitivity in dark conditions with Topo-G over SMILE.18
“ Although LALEX offers other potential benefits, the more optically advanced treatments only available with LASIK currently do seem to have an edge over LALEX in terms of visual results ”
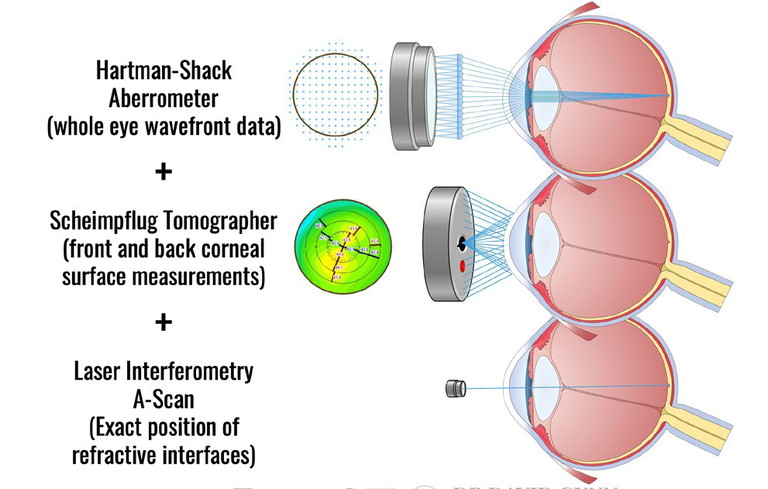
Data collection on the Sightmap device combines three diagnostic devices into one.
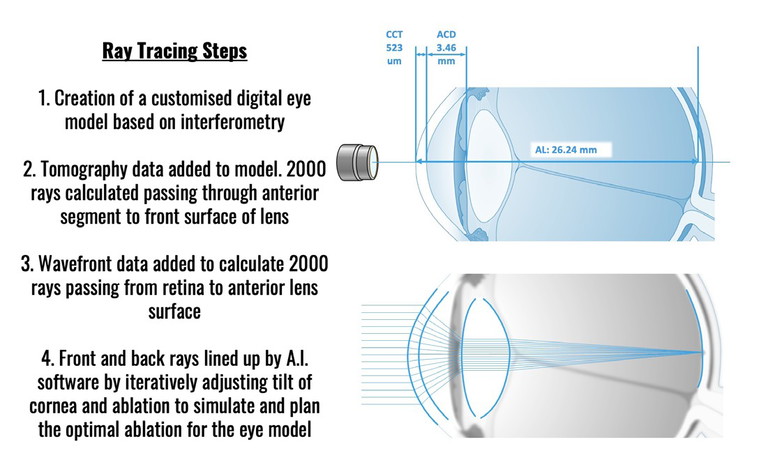
Ray Tracing for Wavelight Plus involves four steps to create a highly customised ablation tailored to each eye.
In another study, 88.9% achieved 6/6 or better with Topo-G compared to 83.7% with SMILE at six months post-operatively, even despite higher attempted cylinder corrections.19 Although LALEX offers other potential benefits, the more optically advanced treatments only available with LASIK currently do seem to have an edge over LALEX in terms of visual results. LALEX volumes are increasing worldwide, particularly in Asia. As these cutting-edge ablation technologies develop on LASIK platforms, they will surely trickle down to LALEX over time as photo disruption technology improves.
RAY TRACING LASIK – A NEW LEVEL OF CUSTOMISATION
Ray tracing technology is not new to the field of optics, but its application in refractive surgery is a novel development.
Traditionally used in physics and computer graphics to render lifelike images by simulating light interactions, ray tracing in ophthalmology involves the computational modelling of light ray paths through the optical system of the eye. It not only considers the corneal surface (as in Topo-G treatments), but also integrates biometric data from the entire eye including the lens and retinal position.
A method was first described in 2008 to simulate a model eye and optimise visual outcomes based on how rays are calculated to traverse these structures.20 This holistic approach would allow for a refined treatment plan that could anticipate and compensate for how alterations to the cornea would affect vision post-surgery, including the correction of complex higherorder aberrations and providing a truly personalised refractive correction.
“ Ray tracing technology is not new to the field of optics, but its application in refractive surgery is a novel development ”
Ray tracing on a patient.
Subsequent research and development of a combination diagnostic device, as well as automated ray tracing algorithms, led to European CE marking of the Sightmap device in 2019 from the Wavelight/Alcon team. The Wavelight Plus treatment (originally called InnovEyes) is the first commercially available system to use this technology and offers an unprecedented level of customisation and precision.
WAVELIGHT PLUS
The core of the Wavelight Plus system’s functionality is the new Sightmap device. This three-in-one combination device contains a Scheimpflug corneal tomographer, a Hartman-Schack wavefront aberrometer, and an interferometry based biometer. The diagnostic data collected by this device is then fed into software that builds a detailed customised model eye that digitally represents the patient’s real eye. It includes the cornea’s front and back surfaces, the lens’s shape and position, and the eye’s length. By simulating how light travels through and interacts with each of these components, the system can predict how changes in corneal shape will affect vision. Complex calculations are undertaken to create a truly customised ablation profile that is highly tailored to each eye’s unique ocular characteristics.20,22
EARLY RAY TRACING RESULTS
The first landmark study of this technology was a multicentre European trial evaluating the safety, efficacy, and predictability of ray tracing LASIK in treating moderate to high myopic astigmatism. This study predated the Sightmap device and Wavelight Plus, and involved collection of data from multiple sources sent offsite to have treatments calculated. It showed results comparable or improved upon to WFO and WFG treatments.21 In 2013, one-year results comparing ray tracing with WFO and Topo-G were published. All ray tracing eyes achieved 6/6 or better uncorrected vision and were more likely than Topo-G and WFO to obtain better than 6/6 vision at 12 months post-procedure. No eyes lost CDVA, 38% gained one line of CDVA, and 10% gained more than one line.22
SIGHTMAP RESULTS
CE approval of the Sightmap device in 2019 meant streamlined data capture and treatment planning were incorporated into the one device. Results after this time represent the current version of the Wavelight Plus algorithm. In 2020, Kanellopoulos reported the first preliminary results from 50 eyes with 100% obtaining 6/6 uncorrected vision, 65% of eyes gaining one line of corrected vision, and 38% two lines of corrected vision with significant improvements in contrast sensitivity at six months postoperatively.23 Two-year results from this group showed a persistence of these outcomes and improvements in higher order aberrations, contrast sensitivity, and subjective quality of vision questionnaires.24 Bala reported his impressive results of 400 eyes in 2023 with 100% of eyes obtaining 6/6, 89% obtaining 6/5, 51% obtaining 6/4.5, and 10% obtaining 6/3.25 Thirty-nine per cent of eyes obtained one line of UDVA better than their preoperative CDVA. Bala determined that there was no clinically significant increase in higher order aberrations, which was similar to the findings by Kanellopoulos, and additionally identified a decrease in spherical aberrations post-operatively.25 An international postmarketing study showed similar results with 98.1% reaching 6/6 or better, 82.5% reaching 6/5 or better, and 99.1% of patients satisfied and happy with their ray tracing femto-LASIK procedure.26 Results that show 83–89% of ray tracing eyes reaching 6/5 are impressive and compare well to other published results of wavefront optimised (72–76%),10 wavefront guided (64–69%),10,27 and topography-guided treatments (22–69%).11,12
EARLY EXPERIENCE IN QUEENSLAND
The Queensland Eye Institute has had early access to the Sightmap device and we performed our first Wavelight Plus procedures in April 2024. At the one-week postop visit, three of 16 eyes treated obtained 6/3 vision, not something we would normally expect with Topo-G treatments.
One excited patient commented, “It’s weird, but I think I can see better now than I could before in my glasses, I don’t think I have ever had vision like this”. It seems that patients can tell the difference, even early. We do not yet have numbers of significance to report outcomes, but we are excited to see how they will compare to the great results we already achieve with Phorcides-guided Contoura (more than 90% of LASIK treatments performed by the author use this method).
CONCLUSION
Conventional LASIK, while effective for a broad range of refractive errors, often falls short in treating complex aberrations or achieving outcomes tailored to the specific characteristics of each eye. The Wavelight Plus system, by contrast, uses ray tracing to precisely map and correct these aberrations and represents a revolutionary step forward in the field of refractive surgery. By harnessing the power of ray tracing, it provides tailored treatments that are likely to significantly improve visual outcomes and patient satisfaction. Longer-term data and results are needed to see if the early results will extend into the general market. As the technology is rolled out, it holds the promise of setting new benchmarks in precision and safety in refractive surgery. Wavelight Plus stands poised to become a pivotal tool in the arsenal of refractive surgeons worldwide.
To earn your CPD hours from this article visit: mieducation.com/a-paradigm-shift-in-refractivesurgery-wavelight-plus-ray-tracing-lasik.
This article is sponsored by Alcon Surgical.
References
1. Pallikaris IG, Papatzanaki ME, Georgiadis A, et al., Laser in situ keratomileusis. Lasers in surgery and medicine. 1990;10(5):463–8. doi:10.1002/lsm.1900100511.
2. Kim TI, Alio Del Barrio JL, Ang M, et al. Refractive surgery. Lancet. May 18 2019;393(10185):2085-2098. doi:10.1016/S0140-6736(18)33209–4.
3. Hashmani S, Hashmani N, Rajani H, et al. Comparison of visual acuity, refractive outcomes, and satisfaction between LASIK performed with a microkeratome and a femto laser. Clin Ophthalmol. 2017;11:1009–1014. doi: 10.2147/OPTH. S137451.
4. Sekundo W, Kunert K, Russmann C, et al. First efficacy and safety study of femtosecond lenticule extraction for the correction of myopia: six-month results. J Cataract Refract Surg. Sep 2008;34(9):1513–20. doi: 10.1016/j. jcrs.2008.05.033.
5. TrinhT, Balamurali A, Choose your words carefully. The Ophthalmologist. 2023. Available at: crstodayeurope.com/articles/mar-apr-2023/standardizing-terms-in-cataract-andrefractive-surgery/ [accessed 6 June 2023].
6. Munnerlyn CR, Koons SJ, Marshall J, Photorefractive keratectomy: A technique for laser refractive surgery. Journal of Cataract and Refractive Surgery. 1988;14(1):46– 52. doi: 10.1016/s0886-3350(88)80063-4.
7. Moreno-Barriuso E, Lloves JM, Barbero S, et al. Ocular aberrations before and after myopic corneal refractive surgery: LASIK-induced changes measured with laser ray tracing. Invest Ophthalmol Vis Sci. May 2001;42(6):1396–403.
8. Wang Y, Zhao KX, Zuo T, et al. Ocular higher-order aberrations features analysis after corneal refractive surgery. Chin Med J (Engl). Feb 20 2007;120(4):269–73.
9. Rabinowitz YS, Yang H, Brickman Y, et al. Videokeratography database of normal human corneas. Br J Ophthal. 1996;80(7):610–616. doi:
10.1136/bjo.80.7.610. 10. Stonecipher KG, Kezirian GM. Wavefront-optimized versus Wavefront-guided LASIK for myopic astigmatism with the Allegretto Wave: Three-month results of a prospective FDA trial. Journal of Refractive Surgery. 2008;24(4):S424–S430. doi: 10.3928/1081597x20080401-20.
11. Stulting RD, Fant BS, et al. T-Cat Study Group. Results of topography-guided laser in situ keratomileusis custom ablation treatment with a refractive excimer laser. J Cataract Refract Surg. 2016;42(1):11–8. doi: 10.1016/j. jcrs.2015.08.016.
12. Kanellopoulos AJ, Topography-modified refraction (TMR): Adjustment of treated cylinder amount and axis to the topography versus standard clinical refraction in myopic topography-guided LASIK. Clin Ophthalmol. 2016;10:2213–2221. doi:10.2147/OPTH.S122345.
13. Motwani M, The use of WaveLight Contoura to create a uniform cornea: The LYRA Protocol. Part 3: the results of 50 treated eyes. Clin Ophthalmol. 2017;11:915–921. doi: 10.2147/OPTH.S133841.
14. Stulting RD, Durrie DS, Potvin RJ, et al. Topographyguided refractive astigmatism outcomes: Predictions comparing three different programming methods. Clin Ophthalmol. 2020;14:1091–1100. doi: 10.2147/OPTH. S244079.
15. Stulting RD, Lobanoff M, Potvin R, et al. Clinical and refractive outcomes after topography-guided refractive surgery planned using Phorcides surgery planning software. J Cataract Refract Surg. Sep 1 2022;48(9):1010– 1015. doi: 10.1097/j.jcrs.0000000000000910.
16. Chen LY, Manche EE, Comparison of femtosecond and excimer laser platforms available for corneal refractive surgery. Curr Opinion Ophthalmol. 2016;27(4):316–22. doi: 10.1097/ICU.0000000000000268.
17. Kanellopoulos AJ, Topography-guided LASIK versus small incision lenticule extraction (SMILE) for myopia and myopic astigmatism: A randomized, prospective, contralateral eye study. J Refract Surg. 2017;33(5):306–312. doi:10.3928/1081597X-20170221-01.
18. Yang L-J, Mi S-J, Sun L, Chen M-X. Early visual function outcomes of topography-guided FS-LASIK and SMILE in treatment of myopia and myopic astigmatism. Int Journal of Ophthal. 2021;14(3):423–429. doi: 10.18240/ ijo.2021.03.15.
19. Schallhorn JM, Seifert S, Schallhorn SC. SMILE, topography-guided LASIK, and Wavefront-guided LASIK: Review of clinical outcomes in premarket approval FDA studies. J Refract Surg. 2019;35(11):690–698. doi: 10.3928/1081597X-20190930-02.
20. Mrochen M, Bueeler M, Donitzky C, Seiler T. Optical ray tracing for the calculation of optimized corneal ablation profiles in refractive treatment planning. J Refract Surg. 2008;24(4):S446–S451. doi: 10.3928/1081597x20080401-23.
21. Schumacher S, Seiler T, Mrochen M, et al. Optical ray tracing–guided laser in situ keratomileusis for moderate to high myopic astigmatism. J Cataract Refract Surg. 2012;38(1):28–34. doi: 10.1016/j.jcrs.2011.06.032.
22. Cummings A, Kelly GE, Optical ray tracing-guided myopic laser in situ keratomileusis: 1-year clinical outcomes. Clin Ophthal. 2013;7:1181. doi: 10.2147/ opth.s44720.
23. Kanellopoulos AJ, Initial outcomes with customized myopic LASIK, guided by automated ray tracing optimization: A novel technique. Clin Ophthal. 2020;14:3955–3963. doi:10.2147/opth.s280560.
24. Kanellopoulos A. Ray tracing customization in myopic and myopic astigmatism LASIK treatments for low and high order aberrations treatment: Two-year visual function and psychometric value outcomes of a consecutive case series. Clin Ophthal. 2024;18:565–574. doi:10.2147/opth.s444174.
25. He G, Bala C, Ray tracing–guided myopic LASIK: real-world clinical outcomes. J Cataract Refract Surg. 2023;49(11):1140–1146. doi: 10.1097/j. jcrs.0000000000001286.
26. Kanellopoulos A, Maus M, Bala C, et al. International multicenter, myopic and myopic astigmatism femto LASIK, customized by automated ray tracing ablation profile calculation: A postmarket study. Clin Ophthal. 2024;18:525–536. doi: 10.2147/opth.s435581.
27. Mrochen M, Kaemmerer M, Seiler T, Clinical results of wavefront-guided laser in situ keratomileusis 3 months after surgery. J Cataract Refract Surg. 2001;27(2):201–207. doi: 10.1016/s0886-3350(00)00827-0. ANZ-WLO-2400003

Dr David Gunn MBBS (Hons I) BSc CertLRS FRANZCO FWCRS is a Brisbane-based ophthalmologist specialising in cornea, cataract, and refractive surgery. He has a research focus in laser eye surgery and advanced keratoconus treatments. He introduced CAIRS surgery to Australia in 2021 and has performed several Australian and world-first procedures in the area of keratoconus. He practises at the Queensland Eye Institute and Focus Vision Clinic.