mieducation
The Raft of New 0.01% Atropine Studies: What They Tell Us for Practice
Published research on the intervention of myopia progression has been increasing at a remarkable rate. However, how we interpret this research into clinical practice remains challenging. Recent publications on atropine 0.01% have reported varying results and conclusions, highlighting questions and interpretations in clinical practice. This article aims to analyse these studies to aid best management of myopia progression in children. A comparison of demographics and results is summarised in Table 1.
WRITERS Dr Loren Rose
LEARNING OBJECTIVES
On completion of this CPD activity, participants should be able to:
1. Better understand the current literature on using atropine drops for myopia intervention,
2. Better understand comparisons for various results using atropine drops,
3. Interpret the relevance of myopia intervention in clinical practice, and
4. Incorporate new studies into clinical practice.
The use of atropine drops to retard myopia in children was cemented with the Singapore Atropine for the Treatment Of Myopia (ATOM) studies 1 and 2. ATOM 1 compared placebo drops in one eye to atropine 1.0%.1 Topical atropine 1.0% reduced the dioptre progression by 0.92 dioptres (D) and 0.40 mm in axial length measured by ultrasound over two years.
ATOM 2 followed by comparing the effect of atropine 0.5%, 0.1%, and 0.01%.2 The dose of atropine 0.01 % was initially planned as the placebo group, but a historical control was used as it was found to have an effect. At two years, this group of participants, aged six to12 years with at least -2D of myopia and less than 1.5 dioptres cylindrical (DC) of astigmatism, progressed between -0.30 to -0.49D and 0.27 to 0.41 mm in axial length (atropine 0.5%, 0.1%, and 0.01% respectively). This showed a dose response to atropine and progression.
Atropine 0.01% was well tolerated with minimal side effects of near blur and glare and comparable efficiency to atropine 0.5%. The publication in 2016 of the ATOM 2 fiveyear results showed minimal rebound when the atropine 0.01% drops were ceased for one year and again helped retard progression the following two years in the group of children still progressing fast after the one-year break.3 Atropine 0.01% became the popular choice for retarding myopia progression in children.
In 2019, the Low concentration Atropine for Myopia Progression (LAMP) study was published. This Hong Kong study compared atropine 0.05%, 0.025%, and 0.01% with placebo drops.4 The study included four to 12 years with at least -1.0D of myopia. Interestingly, the placebo group in the first year had very fast progression, double the placebo group in ATOM 1. LAMP 2, over two years, found that atropine 0.05% had double the efficacy compared to atropine 0.01%.4 The side effect profile was also doseresponse related, but atropine 0.05% was well tolerated. LAMP 3 followed, confirming the findings over three years with rebound also dose-related. The higher the dose, the more rebound with a break in the drops.5 The conclusion was that atropine 0.05% was the preferred dose for fast progression.

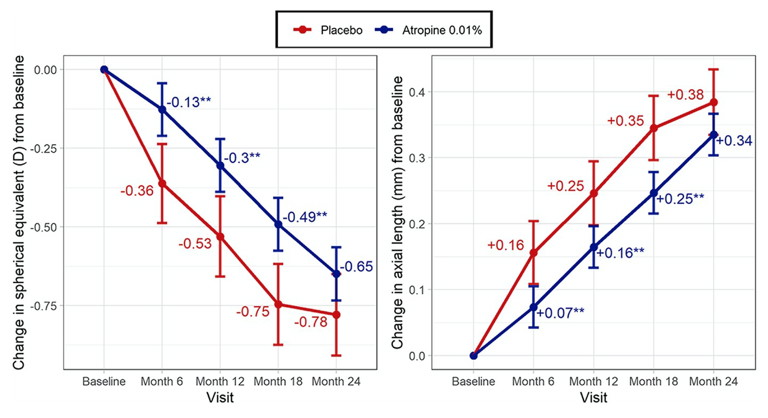
Figure 1. Estimated marginal mean change in spherical equivalent (left) and axial length (right) from baseline from the WA-ATOM study. Statistically different from the placebo group at *p < 0.05 or **p < 0.01. Estimates are adjusted for baseline value; error bars represent standard error.10
Recently, the five-year data for LAMP was published.6 This publication mirrors the ATOM five-year follow-up. The final years four and five all switched to 0.05%, and if participants were on cessation, they were restarted on 0.05% if progressing over -0.5D/year. The results indicate that a rebound at every dose was observed. However, restarting treatment with atropine 0.05% on the fast progressors achieved a similar efficacy to continued treatment. This treatment regime does not correspond to restarting atropine 0.01%, given the study design. However, similar to ATOM 1, restarting atropine treatment (0.01% in ATOM, 0.05% LAMP) seems effective after washout, regardless of the prior concentration used.
The controversy has been that most of these studies, and those that followed, have been based on Asian populations. This has included Japanese,7 Indian,8 and Chinese9 studies, all showing a positive but small effect with the use of atropine 0.01% on retarding spherical equivalent (SE) and axial length (AL) progression.
In the past two years, we have seen the publication of new Western data and the use of atropine 0.01% with conflicting results. Additionally, these studies have had to navigate a global pandemic. This made compliance with the studies difficult. Additionally, the results may have been skewed by the effect of being in lockdown indoors with more near work, both known confounders to myopia progression.
WA-ATOM
The Western Australia-ATOM study (WAATOM) was published in 2022.10 In this study, children six to 16 years, including almost 50% of European descent, compared atropine 0.01% (benzalkonium chloride preservative) to placebo (2:1 ratio).
The children’s inclusion criteria were SE at least -1.5D and less than 1.5DC with at least 0.5D progression in the previous year. At baseline, the placebo group was, on average, a year older than the treatment group. At the one-year mark, there was a 42% reduction in D change and a 36% reduction in AL elongation in the treatment group. The placebo progression was 0.25 mm in the first year, keeping with the ATOM studies.
However, in the two-year follow-up results, atropine 0.01% had an 18% and 11% reduction of D and AL change, which was not statistically significant. The major concern was that the two-year point had many participants drop out of the placebo group. This was statistically significant by the 18-month follow-up (overall dropout was 9.7% in the atropine group and 25% in the placebo group).
Analysis at each six-month endpoint had a statistically significant effect on D and AL until the last six months (Figure 1), with a placebo participant dropout and the placebo left having an unusually low progression rate of 0.03 mm. Additionally, compliance with treatment was not assessed.
Although this study was not powered to detect differences in racial groups, it was noted that the East and South Asian children had minimal effect on D or AL with atropine 0.01%. This contradicts previous metaanalysis finding that atropine treatment is more effective in Asian children.11,12 We await the data following the cessation of drops in the third year, as the long-term rebound effect also requires clarification.
Key message: Australian data demonstrates the positive effect of atropine 0.01% in children six to 16 years who are faster progressors.
CHAMP
In June 2023, the Childhood Atropine for Myopia Progression (CHAMP) study published its three-year data. This was a multicentre, randomised, double-masked, placebocontrolled trial.13 It involved 26 clinical sites in North America and five European countries. Their inclusion criteria were three to 16 years (although most participants were six to 10 years), -0.5 to -6.0D, with less than -1.5DC.
The atropine doses tested were 0.02% and 0.01% compared with a placebo group (ratio 3:2:2). The primary outcome was the proportion of participants responding to the therapy, defined as less than 0.5D progression at three years. This definition is quite a strict criterion assessing response effect. The study design also capped the number of participants older than 11 years to 16, to evaluate progression in the younger population with results corresponding to six to 10 years.
Notably, the baseline data showed participants were mostly low myopes (less than -3D) in all randomised groups, with at least 50% self-identified as racially white. There was also no documented progression prior to the study as inclusion criteria.
Atropine 0.01% was significantly effective at retarding progression measured by both dioptre and axial change at all three endpoints (yearly analysis). Most confusingly, atropine 0.02% had a positive effect at all three endpoints but only significantly with AL and less of an effect than atropine 0.01%. The reduction in progression with atropine 0.01% was 0.24D and 0.13 mm over three years. In calculating the primary outcome, the proportion of participants progressing less than 0.5D progression at three years was 17.5% on placebo, 22.1% on atropine 0.02%, and 28.5% on atropine 0.01%, with only the latter being statistically significant. Each yearly interval analysed demonstrated that atropine 0.02% at one year was similar to atropine 0.01% in response and statistically significant compared to control. At 24 and 36 months, only atropine 0.01% had a significant response defined as very slow progression (Figure 2). All doses were well tolerated. Post hoc analysis did try to explain the discrepancy with atropine 0.02%, with the possible confounder of 23 participants who discontinued trial treatment. Additionally, it was interesting that 17% of the placebo group did not progress greater than -0.5D in a mainly six to 10-yearold population of low myopes. This result reports that there are groups of children with myopia who are slow progressors and do not benefit from intervention.
Key message: European data demonstrates atropine 0.01% has a positive effect on six to 10-year-olds in mixed ethnicity, including racially white. The effect of atropine 0.02% was unclear but had a trend to be helpful. Treatment was not necessary for a group of naturally slow progressors.
PEDIG
Also in 2023, the Pediatric Eye Disease Investigation Group (PEDIG) published the results of a randomised placebo-controlled double-masked trial conducted in 2018–2022 in the USA.14 It included children five to 12 years of age, and compared atropine 0.01% to placebo over two years, followed by six months of no drops to assess rebound. There was also no documented progression prior to the study as inclusion criteria. In this study, 46% were classified as white ethnicity. During the two years, no statistical effect of atropine 0.01% was measured with either D or AL. The data presented for adjusted change in SE and AL showed a trend of atropine having a positive treatment effect, especially early in the trial, but it was not statistically significant. In the following six months, no rebound was documented. This was unsurprising as there was no recordable effect of the preceding medication stopping the medication. This study had better follow-up compared with ATOM2 and LAMP.4
It should be noted that although run through COVID, this study had a slow placebo progression of around 0.8D over two years, which is uncommon in a fast-progressing myope. The initial placebo rate reported in the ATOM 1 study is a more common progression seen in clinical practice of 1.2D progression in two years.1 This might be because no documented progression was required before study inclusion. The ethnicity groups included were approximately 50% white in each group; however, there were about 20% blacks in each group, known to be slow progressors.15 How this may affect the overall progression is unclear.
Key message: Atropine 0.01% did not statistically affect a mixed ethnic race in five to 12 years myopes. This result, with the lowest dose of atropine commonly used, might be explained by no documentation of previous fast progression in the study entry criteria and a large ethnic group known to be slow progressors.
MOSAIC
The Myopia Outcome Study of Atropine In Children (MOSAIC) was published in August 2023.16 Children, six to 16 years of age with at least -0.5D myopic in both eyes and less than 2.5DC, were recruited to either atropine 0.01% or placebo in a 2:1 ratio. This study included predominantly racially white participants (over 80% in the treatment and placebo group). At 12 months, both groups had no statistical difference for SE or AL. At 18 months, the effect of atropine 0.01% was significant, measured by SE and AL. At the 24-month mark, the atropine 0.01% effect was significant for AL and just under significance for SE (Figure 3). Sub-analysis documented a significant treatment effect for SE at 18 months and AL at 18 and 24 months in the white participants.

Figure 2. CHAMP study change in ophthalmic parameters over time.13

Figure 3. Two-year results of daily 0.01% atropine in a European population from the MOSAIC study.16
The analysis of COVID effects in this study noted that the treatment effect was greater if the participant had a low impact from COVID with reduced restrictions. Like all the recent studies, COVID resulted in some difficulties in conducting the studies, as well as lifestyle restrictions known to impact myopia progression.17 Interestingly, atropine 0.01% is known to be the least disease-altering dose and may not have enough effect if a child is a faster progressor in groups such as Asian, younger, or those who do not have enough outdoor exposure. This result notes the need for combined treatment and the message that lifestyle confounders must be addressed to improve treatment outcomes.
Key message: The positive effect of atropine 0.01% was documented in six to 16-year-olds who had documented progression and were predominantly racially white. Although not statistically significant throughout the study, subanalysis noted the effect of COVID-19 on treatment outcomes.
APPLE
The Phase 2 Singaporean APPLE study is a randomised double-masked placebo control comparing the effect of atropine 0.0025%, 0.005%, and 0.01%.18 Consistent with the original ATOM studies, atropine was doseresponsive in its treatment effectiveness. Almost 90% of participants were Asian, and a statistically significant linear dose-response relationship existed between the atropine and placebo groups over 12 months (Figure 4), although atropine 0.0025% did not have a significant treatment effect compared with placebo at 12 months. Subanalysis confirmed that the younger participants progressed faster, and the treatment groups of 0.005% and 0.01% lowered progression, especially in the older and higher baseline myopes.
Key message: Lower doses of atropine than 0.01% may benefit treating myopia progression. Treatment dose may need to be considered as different efficacy was noted depending on age and amount of myopia.
ATLAS
The sobering and ‘food for thought’ ATLAS study19 was published in 2024. This was a double-masked observational study of the longterm outcomes of ATOM 1 and 2. The outcomes were refractive and elongation change and incidence of ocular complication, with 17.8% of ATOM 1 and 39.5% of ATOM 2 participating. Although this is a minority of the original, the follow-up was 10–20 years, providing this group’s first clear long-term data.
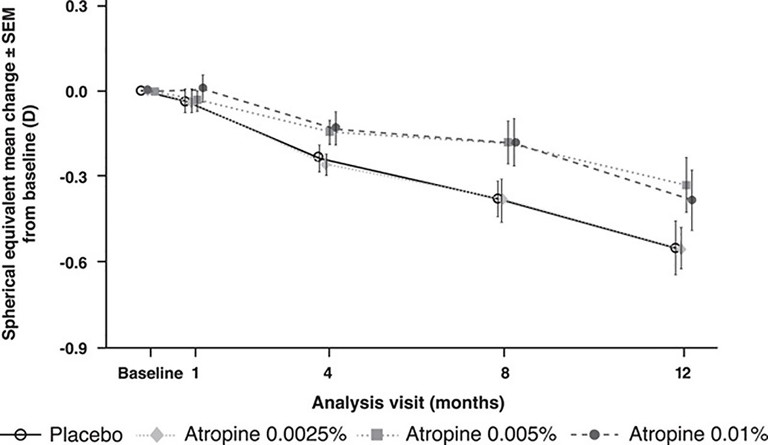
Figure 4. Change in spherical equivalent from baseline to month 12 in the APPLE study.18
Firstly, the short-term use of atropine in children did not impact the final SE or AL between treatment and placebo in ATOM 1or different atropine concentrations in ATOM 2. The greatest progression post-cessation was documented in the younger and high-concentration group.
AL was associated with myopic macular degeneration. There was no other difference in the incidence of cataracts, myopic macular degeneration or parapapillary atrophy when atropine 1% was compared to placebo.
Key message: There is potential for atropine to delay progression and for catch-up to occur when it is ceased, leading to no long-term effect in the end refraction and, importantly, pathological myopia.
SUMMARY
Topical atropine has been extensively documented to reduce myopia progression in children in SE and AL. Most studies demonstrate a relative dose response effect with atropine 0.01% being the least but often statistically most effective dose. A reduction in children progressing less than 0.5D over two years was reported by 65.7% with atropine 1% (ATOM1),1 52.7% with 0.05%, 32.0% with 0.025%, and 22.0% with 0.01% atropine in the LAMP4 studies over two years. Myopia dioptric progression and AL growth were reportedly reduced by 15.9% and 17.5% in the MOSAIC study,16 by 18.0% and 10.5% in the WA-ATOM study,10 and 21.0% and 19.0% in the CHAMP study13 over a two-year interval respectively.
The differences between studies may be due to differences in age and refractive inclusion criteria, differences in ethnicity, documenting previous progression to study entry, and different amounts of confounder effect underscored by COVID-19 during some studies.
Given current knowledge, it is clear that atropine is important in myopia management in children. The effect of the lowest dose often used (atropine 0.01%) may be small in some, but may also be enough to treat their myopia progression and maintain the best treatment to side effect profile for that child. This approach is especially true if there is a summative effect with peripheral defocus glasses or dual-focus contact lenses.
Investigating and documenting a child’s progression and axial growth enables treatment to be tailored to optimise each individual’s outcome. Current trials highlight the need to individualise treatment with the trend to slow growth in older children and lower refractive error, but this is not absolute. Many children are documented to have slow axial growth while young and, vice versa, faster growth when older. Similarly, growth rates vary among different ethnicities but may not follow the expected trend. This knowledge should suggest we can not take a blanket approach to myopia management. In a recent commentary, it was quoted that “clinicians should be able to identify the children at risk of myopia or myopia progression and only implement treatment if there is evidence that the child would benefit from therapy”.20
The long-term effect, or rather lack of effects documented by the ATLAS study, provided knowledge that although atropine treatment was safe over the long term, a short treatment interval may result in no overall change in outcome. So, when considering dose, we must carefully consider the length of time we may need to treat for the best long-term outcome. Clinical trials often treat a defined period to provide outcomes promptly, but to change long-term results, we must treat for longer and be aware of rebound effects. Again, axial change is an accurate and sensitive tool to assess rebound. If we treat all young myopes with higher doses of atropine, to protect them from rebound will we need to treat for longer at this dose? Does this exposse them to greater doses for much longer when some would have been slower progressors prior to treatment if progression was documented first?
The effect of lifestyle, especially time spent outdoors and on near work, must be noted in conjunction with interventive therapies. All treatments may have a reduced efficacy if lifestyle is not addressed. This approach could also mean some children may have good myopia control on lower doses of atropine with less rebound risk when ceasing medication.
Although the data from new studies provide plenty to consider, they also emphasise the need for measured use of topical atropine. The questions: when to start, who to start on, what dose, and when to stop all require further investigation. In clinical practice, the aim should be identifying your fast progressor (at any age), treating with the lowest dose for the desired effect, monitoring to decide if treatment must be titrated to a higher dose, deciding when to stop and then monitoring for treatment rebound. Given the unclear effects of age, refraction, ethnicity and lifestyle on your patient, individualised treatment is paramount.
To earn your CPD hours from this article visit mieducation.com/the-raft-of-new-0.01%-atropine-studies-what-they-tell-us-for-practice.
References available at mieducation.com.

Dr Loren Rose BSc (Hons I) MBBS (Hons) PhD FRANZCO completed her medical degree from the University of Sydney (USyd), graduating with MBBS (Honours). Prior to that, she completed a Bachelor of Science from USyd, graduating with Honours (Class I) in Visual Neuroscience.
Dr Rose completed her ophthalmic training at the Royal Eye and Ear Hospital in Victoria. Following this, she underwent a fellowship in paediatric ophthalmology at the Royal Children’s Hospital, Melbourne. Now based in Sydney, she is a clinical senior lecturer at Macquarie University, an adjunct Associate Professor at the University of Canberra, and she practises privately at Sydney Eyecare Burwood. In 2021, she completed her PhD, titled Myopia Progression in Children, at Macquarie University.