mieducation
Technology to Avoid Pitfalls with Astigmatism and Toric IOLs
Ocular astigmatism is a refractive condition that occurs due to unequal curvatures of the cornea and the crystalline lens, decentration and/or tilting of the lens, unequal refractive indices across the crystalline lens, and in some cases, altered geometry of the posterior pole.1 In the modern world, with advancing technology and digitalisation, a patient’s aim is not just clearer vision with correction, but a more focussed vision with little or no correction. Astigmatism, even as low as 0.5D, can cause fuzzy vision that can impact uncorrected visual acuity (UCVA) outcomes. Astigmatism may be corrected postoperatively with the use of glasses and/or contact lenses. However, most patients undergoing cataract surgery aim for good uncorrected vision, at the least for distance, if not all distances.
In this article, Clinical Associate Professor Smita Agarwal discusses options for the correction of astigmatism and the technology now available to ensure optimal patient outcomes.
WRITER Clinical Associate Professor Smita Agarwal
LEARNING OBJECTIVES
On completion of this CPD activity, participants should be able to:
1. Be aware of the high prevalence of preoperative astigmatism in the adult population,
2. Understand how residual astigmatism post-cataract surgery can affect vision,
3. Understand how toric intraocular lenses treat astigmatism, and
4. Realise the role of technology in enhancing workflows and patient outcomes.
Astigmatism management at the time of cataract surgery should be addressed by all surgeons to ensure optimal postoperative vision. It is estimated that 37.5% of cataract patients have astigmatism of >1D2 and 19–22% have astigmatism of >1.5D3–5 before undergoing surgery. Currently, intraoperative techniques being used to treat astigmatism are: clear corneal incisions (CCI) on the steepest meridian, paired opposite clear corneal incisions (POCCIs) on the steepest meridian, corneal relaxing incisions, and toric intraocular lens (IOL) implantation.
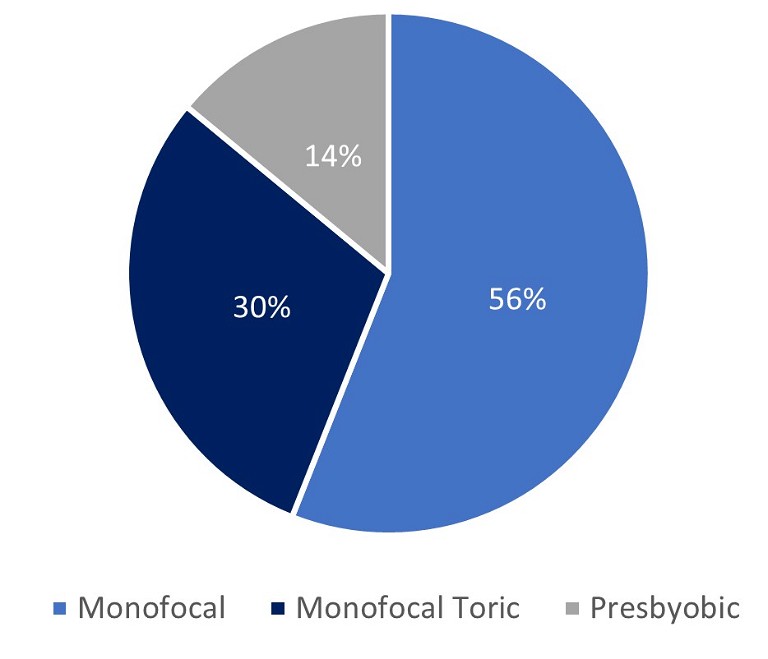
Figure 1. IOL modality in Australia.
However, incisional techniques do not offer the predictability and stability provided by a toric IOL.6 Toric IOL implantation can correct pre-existing astigmatism as low as 0.75D and is the method of choice to correct high levels of astigmatism. Due to the current healthcare landscape, Australian astigmatic patients are fortunate to have access to the most accurate method of correction. This sees Australia with a toric implantation rate close to 30%,7 which reflects the prevalence of visually significant corneal astigmatism noted above.
The power of the required IOL is accurately determined with the use of ocular biometry, which measures axial length of the eye and curvature of the cornea. These parameters, along with a few others, are then used with various formulas to determine the optimal IOL power for an individual eye to achieve the desired refractive outcome.
Although toric IOLs are a very safe and effective way to manage astigmatism with cataract surgery, success depends not only on accurate preoperative measurements performed on a healthy ocular surface, but also on the stability and alignment of the toric IOL along the intended axis and capsule overlap on the IOL optic. Other factors that have a potential role include IOL material, adequate viscoelastic removal from behind the lens during cataract surgery, and axial length of the eye. Rotational stability is crucial, and realignment is needed when rotation is more than 10° from the target axis.8 It is vital that the values are in agreement across all modalities, which can include auto-refractor, glasses prescription, biometer, topographer, and/or tomographer.
Most patients with astigmatism understand, accept, and usually welcome the idea of correction with cataract surgery, as they have been previously corrected with their glasses and/or contact lenses. However, there are a few cases in which a patient’s corneal astigmatism has been negated by their natural lenticular astigmatism and they have never had correction in their glasses and/or contact lenses. These patients need more chair time and extra testing to demonstrate the need for a toric IOL – especially when they insist that they never had astigmatism.
MEASUREMENT OF CORNEAL ASTIGMATISM
Small amounts of postoperative residual astigmatism – even as little as 0.5D – can significantly impact visual outcome, resulting in unsatisfied patients.9 Common ocular surface conditions, like dry eye disease (DED), epithelial basement membrane dystrophy (EBMD), Salzmann nodular degeneration (SND), and pterygium, can cause significant irregular astigmatism and higher-order aberrations.
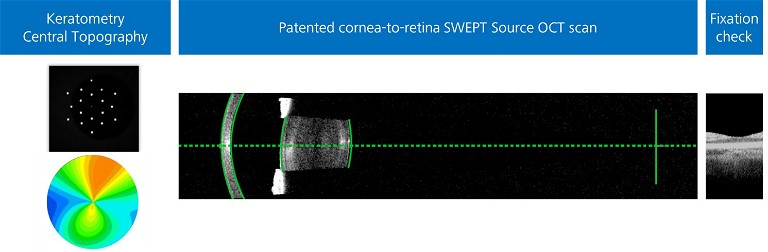
Figure 2. A swept-source OCT scan highlighting the importance of accurate keratometry, topography, and fixation check by visualisation of the foveal pit.
There are multiple devices available, based on different technologies, to measure corneal astigmatism. These include a manual keratometer, automated keratometer, placidobased keratometer, point source colour LED topographer, Scheimpflug image-based topographer, low-coherence reflectometer, and scanning slit corneal topographer.10–15 Because each device has its own characteristics, including different refractive indices and measurement areas, measurements obtained from different devices may not be comparable. It is important, therefore, to evaluate the quality of each measurement before using it to plan the surgery.
Measurements can also be influenced by unstable tear film, ocular surface disease etc.16,17 More than half of patients are likely to have meibomian gland dysfunction (MGD), which is not obvious but can get worse or become obvious after surgery. 18,19 Optimising the ocular surface prior to cataract surgery will contribute to more successful outcomes.
Even with advances in technology and ocular surface optimisation techniques, the axes of astigmatism measured using different devices can be highly variable.20 Incorrect position of the patient’s head, low lying eye lids, or dry eyes while performing keratometry may be possible reasons for variable keratometric readings.16
It has been suggested that using the average of manual and automated keratometer measurements reduces the outliers for precise results. It is important to note that axis location and magnitude of astigmatic measurement should be in alignment with at least three different methods, including manual, automated keratometry, and topography/tomography. If no two measurements are consistent, try optimising the ocular surface then repeating the measurement – or avoid using a toric IOL.
Several devices have been developed to measure corneal astigmatism for preoperative planning. The anterior corneal surface can be accurately measured using various technologies and remains an important variable in toric IOL calculations. Some instruments, like Argos and Verion Image Guided System (Alcon Laboratories, Inc, Fort Worth, TX, USA), use only anterior corneal measurements for the calculations. Others, like Pentacam (Oculus Optikgerate GmbH, Wetzlar, Germany) or Galilei (Zeimer Ophthalmic Systems AG, Port, Switzerland), use an integrated value of anterior and posterior power of the cornea.21
“ Even with advances in technology and ocular surface optimisation techniques, the axes of astigmatism measured using different devices can be highly variable ”
Conventionally, only the anterior corneal surface was measured, assuming that the posterior cornea does not contribute much to refractive astigmatism.22 However, recent studies have demonstrated that both the anterior and posterior corneal astigmatism (PCA) contribute to the total corneal astigmatism (TCA), and ignoring posterior corneal measurements may induce errors in calculations of astigmatic treatment. 23,24 If posterior corneal astigmatism is not taken into account, then it may overestimate with the rule (WTR) astigmatism by 0.5–0.6D and underestimate against the rule (ATR) astigmatism by 0.2–0.3D.22 Ignoring posterior corneal astigmatism can also produce an axis error of 7.4±10.3°.25
Posterior corneal astigmatism can be predicted using measurements of anterior corneal astigmatism and fixed anterior to posterior corneal thickness ratios, mathematical models, and algorithms derived from healthy eyes.22 Various nomograms, adjustment coefficients, and calculators can be used to factor posterior corneal astigmatism into anterior cornea measurements.26–29 Koch et al. and Goggin et al. formulas can be used to select toric IOL powers for astigmatism correction, which factor in PCA where only anterior corneal measurements are available. 22,27 These methods may improve accuracy but remain inherently inaccurate due to the lack of vector analysis to calculate the TCA, while the Abulafia-Koch formula and Barrett’s toric calculator provide net astigmatism using vector analysis. 28,29 Barrett’s toric calculator uses the Universal II formula to calculate effective position of the lens and predicts PCA based on a theoretical model to provide toric IOL power. 30
Recently, to obtain net corneal power measurement, intraoperative methods were made available, such as the ORA (Alcon Laboratories, Inc.) and the Holos (Clarity medical systems, Inc., Pleasanton, CA, USA). Despite multiple variables, such as eye lid speculum pressure, intraocular pressure, corneal hydration, and the viscoelastic used to fill the anterior chamber, which can influence net corneal measurements, several studies have shown promising results.31
To avoid multiple devices and maintain consistency and accuracy, the posterior corneal surface can also be directly imaged to measure PCA in each patient using the IOLMaster 700 (Carl Zeiss Meditec AG, Jena, Germany) with total keratometry (TK). It combines its well-known three zone keratometry with swept-source optical coherence tomography (SS-OCT) technology to determine the anterior and posterior corneal surface. This information is used to generate the TK value and central topography maps. Given the TK value is compatible with existing keratometry data, on which IOL calculation formulas are based, it can be incorporated into current non-toric and toric IOL formulas.32 It can also be used in formulas specifically developed to use TK values, such as Barret TK Universal II. The Barrett toric calculator is considered a reliable and accurate tool for preoperative planning and has consistently been shown to produce low 29,33–35 residual astigmatism prediction errors.
Either predicted or measured PCA values can be used to perform toric IOL calculations that significantly reduce postoperative refractive errors.29,36–38 Recently, in an evaluation of 602 eyes, it was demonstrated that accuracy of residual astigmatism prediction is improved using the Barrett toric calculator with measured PCA from the IOLMaster 700 rather than predicted PCA.39 Furthermore, direct measurement of the posterior corneal surface to determine total corneal power improves the accuracy of outcomes in post-refractive surgery eyes.40
For a surgeon to have further confidence in accuracy of corneal measurements, patients are required to fixate correctly while measurements are acquired along the visual axis. Incorrect fixation can lead to incorrect axial length and keratometry measurements, which can increase the risk of refractive surprises. Some devices, such as the IOLMaster 700, have a fixation check. This enables visualisation of the foveal pit to check the patient is fixating, which has the added benefit of screening overt macular pathologies.

Figure 3. A Zeiss cataract workflow connects devices and applications between clinic and theatre.
NATURE/TYPE OF ASTIGMATISM
Understanding corneal shape and characteristics of astigmatism is as important as the magnitude of astigmatism. Therefore, it is important to screen all patients for any irregular corneal topography. While the above dedicated corneal topographers provide a more detailed analysis of the cornea, the integrated central topography maps on the IOLMaster 700, which cover the central 4mm of the cornea, also provide detail about the nature of astigmatism and the visually relevant area of the cornea. This is relevant when planning for cataract surgery and considering the type of IOL. Toric IOLs should be considered with caution in patients with irregular astigmatism. Pinhole implants can be implanted in patients with irregular astigmatism to help with dysphotopsia and photophobia.41
OPTIMISING ASTIGMATIC PATIENT MANAGEMENT
Considerable time is dedicated to planning a toric IOL for an astigmatic patient, particularly a multifocal toric IOL. Therefore, using technology that offers workflow efficiency without compromising on accuracy is paramount. Having an integrated instrument that gives accurate, reliable, and consistent complete data is much more efficient than using multiple pieces of equipment to obtain similar data. Further, supporting the system with an IOL calculation and ordering platform that mitigates against transcriptions errors not only protects the accuracy of the data, it also brings more efficiency to a practice, thereby allowing more time spent counselling the patient.
The Zeiss cataract workflow aims to connect devices and applications between clinic and theatre, to create efficiency without compromising patient outcomes.
From the IOLMaster and other devices, data is transferred automatically to EQ Workplace, a surgical planner that populates relevant fields for IOL calculation, selection, and ordering. This can be done onsite from the device or remotely.
Connected to the Callisto Eye markerless alignment system (Carl Zeiss Meditec AG) in the theatre, all surgical assistance functions are available and pre-set, saving surgeons and nurses valuable theatre time, and protecting against transcription errors. A reference image from the IOLMaster is transferred to Callisto Eye and will automatically overlay in the eyepiece for precise toric alignment. Manual preoperative markings can be skipped.
“ Toric IOLs should be avoided in patients with irregular astigmatism, zonular instability, and in patients with severe dry eye not responding to treatment ”
Additionally, with the separately available Zeiss EQ Mobile app, biometric and surgical planning data automatically uploads to the cloud for remote access. This is a practical option for those working between several locations as it allows for patient data transfer in the theatre. The latest addition to the cataract workflow – the Quatera 700 phaco device and its new pump system – is also digitally connected, displaying relevant information and video feed from the surgery.
IOL CALCULATION FORMULAS
Traditionally, the methods or formulas to calculate the power of an IOL have been called as first, second, third, and fourth generation. The third, and fourth are more recent and cover a wider spectrum of eye lengths compared to older formulas. Being more advanced, the modern formulas yield fewer errors in prediction and incorporate more variables.
In 2017, Ferreira et al33 compared results from several toric calculators and regression formulas to determine which methods were most accurate and yielded the lowest predicted residual astigmatism. The Barrett toric calculator had the lowest centroid prediction errors. Since then, the Barrett formula has consistently demonstrated high accuracy across a range of axial lengths and corneal shapes, including post-refractive corneas. More recently, the Kane toric formula, incorporating artificial intelligence, has also demonstrated superior performance for all axial lengths.42
Modified formulas also exist for keratoconic corneas, which are important for corneas with irregular astigmatism. Fortunately, several biometric devices or integrated cataract planning systems now incorporate a wide range of the IOL calculation formulas. This is advantageous over the online toric IOL calculators, which require nursing staff to manually enter patient variables, thereby increasing the possibility of typographical/ transcription errors.
TORIC IOLS
Lake et al.43 found that toric IOLs gave a higher chance of achieving a residual astigmatism of less than 0.50D when compared with limbal relaxing incisions. Refractive outcomes are not only dependent on precise measurement of preoperative corneal astigmatism, accurate and reliable calculation of the axis, and power of the toric IOL to be implanted – they are also dependent on optimal positioning of the high quality toric IOL during cataract surgery.44
Toric IOLs should be avoided in patients with irregular astigmatism, zonular instability, and in patients with severe dry eye not responding to treatment. Despite ongoing advances, a review of the literature reveals that there is high variability in visual outcomes due to several factors affecting preoperative planning, surgical technique, and postoperative IOL rotation.
Surgically induced astigmatism (SIA) should be incorporated to achieve tighter results, although the effect of SIA are minimal if clear corneal temporal incisions of less than 2.4mm are being made.45
Alignment of Toric IOLs
Accurate alignment of the toric IOL intraoperatively, after correcting for any cyclo-rotation, is key to achieving good refractive outcomes. This can be achieved by using manual presurgical ink marks placed on the corneal limbus while the patient is sitting in an upright position, with iris pattern references, a three-point corneal marker and/ or pendulum marker.
Refractive accuracy can be affected if there is insufficient visibility due to dissolution of ink marks and/or imprecise marking. To overcome these issues, image guided systems, such as Callisto Eye and Verion are helpful.
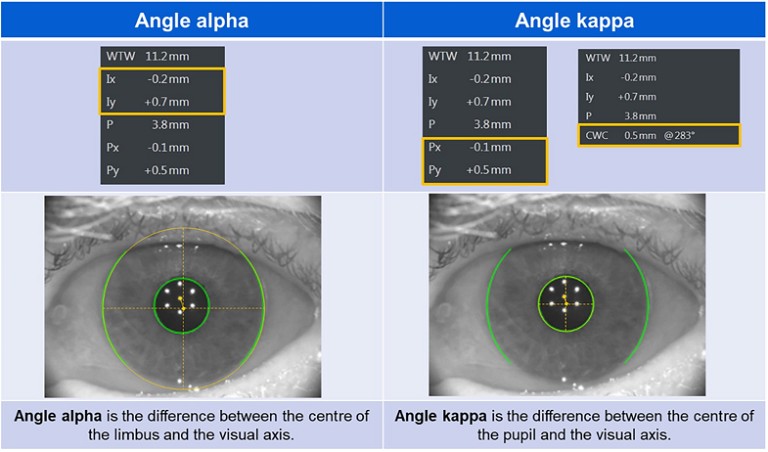
Figure 4. Angle alpha and angle kappa.
Callisto Eye does not require an additional planning station for transfer of data from biometry to the surgical microscope. The data is either transferred via USB from IOLMaster, or it can be transferred automatically via EQ Mobile, thereby avoiding any translational errors and also enhancing efficiency. Conveniently designed to be used directly under the microscope, it does not require an external image divider, such as that needed with the Verion system.
“ Poor alignment of a toric IOL with visual axis may induce astigmatism, reduce the amount of correction, and affect visual quality ”
Stability of Toric IOLs
Toric IOLs can rotate after implantation, especially in the immediate postoperative period, with the toric offset or tilt inducing higher-order aberrations which may negatively impact visual outcome.46 This occurs due to various factors including retained viscoelastic behind the lens, postoperative changes in pressure (hypotonia) that destabilise the anterior chamber, capsulorhexis size and centration, capsule overlap on optic, design and material of the toric IOL, axial lengths >24mm and a large capsular bag.47 The longer the axial length of the eye, the greater the size of the capsular bag, which may decrease IOL stability due to reduced equatorial friction on the lens.48
Rotational stability may also be affected in patients with high myopia and potentially weaker zonules,49 and with smaller diameter IOLs.50 If the IOL rotates by 30°, the astigmatism remains unchanged and aligned at a meridian different from the original steep meridian.
The few studies that compare rotational stability with IOL material and design, have found comparable stability with open-loop hydrophobic acrylic IOLs and plate haptic hydrophilic acrylic IOLs.51 Well-centred IOLs in the capsular bag and an anterior capsule overlap help with stability. It has also been suggested that epithelial cells of the anterior capsule should be left in situ while implanting a toric IOL. This is to avoid any free space between the capsule and lens as a result of fibrotic contraction.49
Centration of Toric IOLs
Good centration of an IOL is essential to achieve optimal visual outcomes, and particularly important with toric multifocal IOLs to avoid patient dissatisfaction due to the risk of higher-order aberrations. Poor alignment of a toric IOL with visual axis may induce astigmatism, reduce the amount of correction, and affect visual quality.
Image guided systems further help in guiding and aligning the lens within the bag at the visual axis. It is important to measure angle alpha (distance between the visual axis and the centre of the limbus) and angle kappa (distance between the visual axis and the centre of the pupil) prior to using premium IOLs, like toric and toric multifocals, for good visual outcomes. In the presence of slightly higher angle kappa it might be useful to nudge the IOL slightly nasally for better centration, but it is preferrable to avoid multifocal lenses (toric or non-toric) when the angle kappa and alpha measurements are high (usually >0.4mm).52
RESIDUAL ASTIGMATISM
Despite the use of advanced technology and the best efforts to preoperatively assess, plan, and perform cataract surgery, a recent meta-analysis found that the average residual astigmatism following toric IOL implantation ranges from 0.18 to 0.77D.53 Resultant astigmatism should be corrected if it is enough to cause patient dissatisfaction with blurred vision, diplopia, photophobia, and dysphotpsia.54
Refractive surprise is multifactorial, including incision size, incision effect, IOL power and IOL alignment. To assist in management of residual astigmatism, online astigmatism fix calculators are available, such as ToricPro by Goggin and LaHood, the Barrett Rx formula and the Berdhal and Hardten calculator.55–57 These check for rotational analysis and calculate any required rotation, which may reduce postoperative astigmatism.
Residual astigmatism can also be corrected with glasses/contact lenses, corneal ablation procedures (LASIK/PRK), arcuate keratotomy and IOL exchange in cases of high refractive surprise. Piggy-back IOLs can be considered if patients are not suitable for either corneal ablation or IOL exchange.
CONCLUSION
Cataract surgery is not just the removal of a cataract and replacement with an IOL. It has become a branch of refractive surgery with patients wanting a visual outcome to suit their lifestyle.
The prevalence of preoperative astigmatism has been reported to be 86.6%, of which 35–40% of patients have astigmatism of ≥1.0D and 19–22% patients of ≥1.5D.4 Patients notice deterioration of visual quality if residual astigmatism exceeds three quarters of a diopter, with patients that receive toric, toric multifocal, and toric extended depth of focus IOLs being less tolerant of symptoms.
Currently, toric IOLs are considered the most accurate form of astigmatism correction during cataract surgery and, with advanced technology, errors can be minimised with better alignment and centration resulting in happier and more satisfied patients.
“ Piggy-back IOLs can be considered if patients are not suitable for either corneal ablation or IOL exchange ”
References
1. Read, S.A., Collins, M.J., Carney, L.G., A review of astigmatism and its possible genesis. Clin Exp Optom 2007;90:5–19.
2. Market Scope (2013). 2013 Comprehensive Report on Global IOL Market available at: market-scope.com/cataract-reports [accessed June 2023].
3. Ferrer-Blasco, T., Montes-Mico, R., Peixoto-de-Matos, S.C., et al., Prevalence of corneal astigmatism before cataract surgery. J Cataract Refract Surg 2009;35:70–75.
4. Khan, M.I., Muhtaseb, M., Prevalence of corneal astigmatism in patients having routine cataract surgery at a teaching hospital in the United Kingdom. J Cataract Refract Surg 2011;37:1751–1755.
5. Michelitsch, M., Ardjomand, N., Vidic, B., et al., Prevalence and age-related changes of corneal astigmatism in patients before cataract surgery. Ophthalmologe 2017;114:247–251.
6. Hirnschall, N., Gangwani, V., Crnej, A., et al., Correction of moderate corneal astigmatism during cataract surgery: toric intraocular lens versus peripheral corneal relaxing incisions. J Cataract Refract Surg 2014;40:354–361.
7. Goggin, M., Toric intraocular lenses: evidence-based use. Clin Exp Optom 2022;50:481–489.
8. Tognetto, D., Perrotta, A.A., Bauci, F., Rinaldi, S., Quality of images with toric intraocular lenses. J Cataract Refract Surg 2018;44:376–381.
9. Schallhorn, S.C., Hettinger, K.A., Pelouskova, M., Effect of residual astigmatism on uncorrected visual acuity and patient satisfaction in pseudophakic patients. J Cataract Refract Surg 2021;47:991–998.
10. Shirayama, M., Wang, L., Weikert, M.P., Koch, D.D., Comparison of corneal powers obtained from four different devices. Am J Ophthalmol 2009;148:528.e521–535.e521.
11. Shirayama, M., Wang, L., Koch, D.D., Weikert, M.P., Comparison of accuracy on intraocular lens calculations using automated keratometry, a Placido-based corneal topographer, and a combined Placido-based dual schleimpflug corneal topographer. Cornea 2010;29: 1136–1138.
12. Aramberri, J., Araiz, L., Garcia, A., et al., Dual vs single Scheimpflug camera for anterior segment analysis: precision and agreement. J Cataract Refract Surg 2012;38:1934–1949.
13. Reinstein, D.Z., Gobbe, M., Archer, T.J., Anterior segment biometry: a study and review of resolution and repeatability data. J Refract Surg 2012;28:509–520.
14. Hoffmann, P.C., Abraham, M., Hirnschall, N., Findl, O., Prediction of residual astigmatism after cataract surgery using swept source Fourier domain optical coherence tomography. Curr Eye Res 2014;39:1178–1186.
15. Ventura, B.V., Al-Mohtaseb, Z., Wang, L., et al., Repeatability and comparability of corneal power and corneal astigmatism obtained from a point-source colour light-emitting diode topographer, a Placido-based corneal topographer, and a low-coherence reflectometer. J Cataract Refract Surg 2015;41:2242–2250.
16. Epitropoulos, A.T., Matossian, C., Berdy, G.J., et al., Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg 2015;41:1672–1677.
17. Lee, H., Kim, T.I., Kim, E.K., Corneal astigmatism analysis for toric intraocular lens implantation: precise measurements for perfect correction. Curr Opin Ophthalmol 2015;26:34–38.
18. Alghamdi, Y.A., Mercado, C., McClellan, A.L., et al., Epidemiology of meibomian gland dysfunction in an elderly population. Cornea 2016;35:731–735.
19. Cochener, B., Cassan, A., Omiel, L., Prevalence of meibomian gland dysfunction at the time of cataract surgery. J Cataract Refract Surg 2018;44:144–148.
20. Asena, L., Gungor, S.G., Akman, A., Comparison of keratometric measurements obtained by the Verion image guided system with optical biometry and auto-keratorefractometer. Int Ophathlmol 2017;37:391–399.
21. Davison, J.A., Potvin, R., Refractive cylinder outcomes after calculating toric intraocular lens cylinder power using total corneal refractive power. Clin Ophthalmol 2015;9:1511–1517.
22. Koch, D.D., Jenkins, R.B., Weikert, M.P., Correcting astigmatism with toric intraocular lens: effect of posterior corneal astigmatism. J Cataract Refract Surg 2013;39:1803–1809.
23. Nemeth, G., Berta, A., Lipecz, A., Evaluation of posterior astigmatism measured with scheimpflug imaging. Cornea 2014;33:1214–1218.
24. Savini, G., Versaci, F., Vestri, G., Influence of posterior corneal astigmatism on total corneal astigmatism in eyes with moderate to high astigmatism. J Cataract Refract Surg 2014;40:1645–1653.
25. Ho, J.D., Tsai, C.Y., Liou, S.W., Accuracy of corneal astigmatism estimation by neglecting the posterior corneal surface measurement. Am J Ophthalmol 2009;147:788–795. e781–782.
26. Koch, D.D., Ali, S.F., Weikert, M.P., et al., Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg 2012;38:2080–2087.
27. Goggin, M., Zamora-Alejo, K., Esterman, A., van Zyl, L., Adjustment of anterior corneal astigmatism values to incorporate the likely effect of posterior corneal curvature for toric intraocular lens calculation. J Refract Surg 2015;31:98–102.
28. Abulafia, A., Hill, W.E., Franchina, M., Barrett, G.D., Comparison of methods to predict residual astigmatism after intraocular lens implantation. J Refract Surg 2015;31:699–707.
29. Abulafia, A., Koch, D.D., Wang, L., et al,. New regression formula for toric intraocular lens calculation. J Cataract Refract Surg 2016;42:663–671.
30. Barrett, G.D., An improved universal theoretical formula for intraocular lens power prediction. J Cataract Refract Surg 1993;19:713–720.
31. Hatch, K.M., Woodcock, E.C., Talamo, J.H., Intraocular lens power selection and positioning with and without intraoperative aberrometry. J Refract Surg 2015;31:237–242.
32. Fabian, E., Wehner, W., Prediction accuracy of total keratometry compared to standard keratometry using different intraocular lens power formulas. J Refract Surg 2019;35:362–368.
33. Ferreira, T.B., Ribiero, P., Ribeiro, F.J., O’Neill, J.G., Comparison of astigmatic prediction errors associated with new calculation methods for toric intraocular lenses. 2017. 43:340–347.
34. Abulafia, A., Barrett, G.D., Kleinmann, G., et al., Prediction of refractive outcomes with toric intraocular lens implantation. J Cataract Refract Surg 2015;41:936–944.
35. Kern, C., Kortum, K., Muller, M., et al., Comparison of two toric IOL calculation methods. J Ophthalmol 2018;2018:2840246.
36. Eom, Y., Rhim, J.W., Kang, S.Y., et al., Toric intraocular lens calculations using ratio of anterior to posterior corneal cylinder power. Am J Ophthalmol 2015;160:717–724.e712.
37. Savini, G., Naeser, K., An analysis of the factors influencing the residual refractive astigmatism after cataract surgery with toric intraocular lenses. Invest Ophthalmol Vis Sci 2015;56:827–835.
38. Canovas, C., Alarcon, A., Rosen, R., et al., New algorithm for toric intraocular lens power calculation considering the posterior corneal astigmatism. J Cataract Refract Surg 2018;44:168–174.
39. Wang, L., Koch, D.D., Comparison of accuracy of a toric calculator with predicted vs measured posterior corneal astigmatism. J Cataract Refract Surg 2023;49:29–33.
40. Lawless, M., Jiang, J.Y., Hodge, C., et al., Total keratometry in intraocular lens power calculations in eyes with previous laser refractive surgery. Clin Exp Optom 2020;48:749–756.
41. Agarwal, S., Thornell, E.M., Cataract surgery with a small-aperture intraocular lens after previous corneal refractive surgery: visual outcomes and spectacle independence. J Cataract Refract Surg 2018;44:1150–1154.
42. Kane, J.X., Connell, B., A comparison of the accuracy of six modern toric intraocular lens formulas. Ophthalmology 2020;127:1472–1486.
43. Lake, J.C., Victor, G., Clare, G., et al., Toric intraocular lens versus limbal relaxing incisions for corneal astigmatism after phacoemulsification. Cochrane Database Syst rev 2019;12:CD012801.
44. Hirnschall, N., Findl, O., Bayer, N., et al., Sources of error in toric intraocular lens power calculation. J Refract Surg 2020;36:646–652.
45. Alio, J., Rodriguez-Prats, J.L., Galal, A., Ramzy, M., Outcomes of microincision cataract surgery versus coaxial phacoemulsification. Ophthalmology 2005;112:1997–2003.
46. He, L., Applegate, R.A., Predicting crystalline lens fall caused by accommodation from changes in wavefront error. J Cataract Refract Surg 2011;37:1313–1322.
47. Alpins, N.A., Vector analysis of astigmatism changes by flattening, steepening and torque. J Cataract Refract Surg 1997;23:1503–1514.
48. Shah, G.D., Praveen, M.R, Vasavada, A.R., et al., Rotational stability of a toric intraocular lens: influence of axial length and alignment in the capsular bag. J Cataract Refract Surg 2012;38:54–59.
49. Zhu, X., He, W., Zhang, K., Lu, Y., Factors influencing one-year rotational stability of AcrySof toric intraocular lenses. Br J Ophthlmol 2016;100:263–268.
50. Chang, D.F., Early rotational stability of the longer Staar toric intraocular lens: fifty consecutive cases. J Cataract Refract Surg 2003;29:935–940.
51. Scialdone, A., De Gaetano, F., Monaco G., Visual performance of two aspheric toric intraocular lenses: comparative study. J Cataract Refract Surg 2013; 39:906–914.
52. Holladay, J.T., Apparent chord mu and actual chord mu and their clinical value. J Cataract Refract Surg 2019;45:1198–1199.
53. Kessel, L., Andresen, J., Tendal, B., et al., Toric intraocular lenses in the correction of astigmatism during cataract surgery: A systematic review and meta-analysis, 2016. Ophthalmology 2016;123:275–286.
54. Alpins, N.A., Ong, J.K., Stamatelatos, G., Refractive surprise after toric intraocular lens implantation: graph analysis. J Cataract Refract Surg 2014;40:283–294.
55. APACRS. Barrett Rx formula. Available at: calc.apacrs. org/barrett_rx105 [accessed 23 May 2023].
56. Goggin, M., LaHood, B., TORICpro. Available at toricpro. com [accessed 23 May 2023].
57. Charma, A.C., Khetan, A., Comparing IOLM700 TK, Berdhal and Hardten astigmatism fix calculator and Barrett Rx formula in managing residual astigmatism due to toric intraocular lens misalignment. Indian J Ophthalmol 2022;70:413–419
Clinical Associate Professor Smita Agarwal is a comprehensive ophthalmologist based on the South Coast of NSW, Australia.
She is a former Head of Ophthalmology Department at Shellharbour and Wollongong Public Hospitals in NSW. She is a Clinical Associate Professor at the Graduate School of Medicine, University of Wollongong and lectures at the University of Sydney.
Dr Agarwal specialises in cataract and refractive surgery and has interest in anterior segment disease including keratoconus and management of presbyopia and has experience with almost all the available intraocular lenses.