The MYOPIA ISSUE
mistory
Strong Foundations
and Fresh Research
in Myopia Management

Figure 2. Diffusion Optics Technology (DOT) spectacles.3 Open access.
As this year draws to a close, it is mind boggling to consider how far our knowledge and practice of myopia management has come. This article is an exploration of the latest knowledge of the myopia management landscape and myopia control treatments, with an eye to current clinical applications and future discoveries.
WRITER Dr Kate Gifford
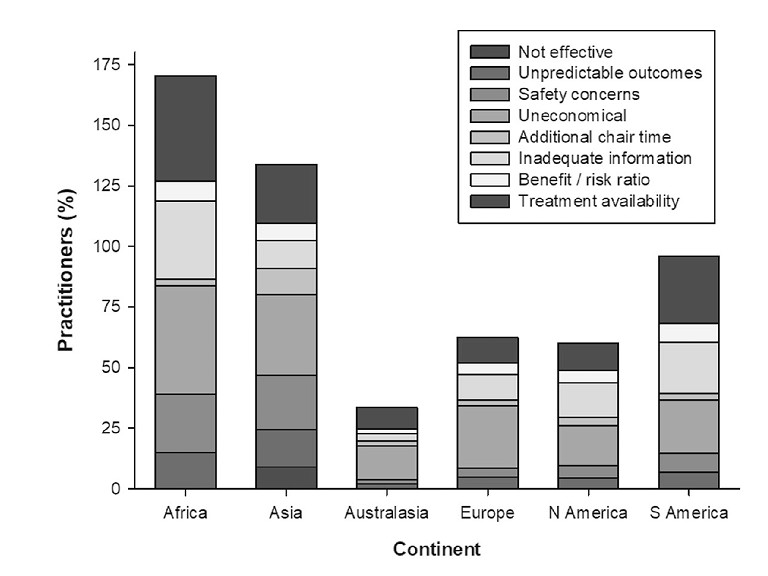
Figure 1. Australasian eye care practitioners cite the lowest number of barriers to uptake of clinical myopia management.6 Open access with the original caption ‘Factors cited by practitioners in different continents for not adopting myopia control approaches. N = 3017’.
“ we can be proud that in our corner of the world we have the lowest rates of prescribing single vision spectacles to children with progressing myopia ”
PRESCRIBING INSIGHTS
This year, 2023, has seen publication of the third volume of International Myopia Institute (IMI) reports,1 following on from previous volumes in 2019 and 2021. The IMI brings together almost 100 academic, clinician, industry, and other stakeholders to generate important consensus papers on the myopia evidence base. The latest volume of reports included the 2023 Digest, providing up-to-date highlights in each of the topics reported upon in the previous volumes.
Full-length papers in this third series cover further key clinical and scientific topics on myopia from adult onset and progression to high myopia in young children, and a thirdedition global survey of myopia management attitudes and strategies in clinical practice.
You can read more about the 2023 IMI reports on page 57 of this issue of mivision.
On the latter, we can be proud that in our corner of the world we have the lowest rates of prescribing single vision spectacles to children with progressing myopia, at less than 20%. This compares with around 40% in North America, Europe, and Asia, and almost 60% in South America and Africa. We have the highest prescribing rates for myopia control spectacle lenses (22%), soft contact lenses (18%), and atropine (13%). Our colleagues in Asia and Europe prescribe slightly more orthokeratology than we do in Australasia. We have a strong understanding of effectiveness of interventions, and rate ourselves as highly active in clinical myopia management. We also cite the lowest number of barriers for uptake of clinical myopia management (Figure 1). But, there was a lower response rate to the 2022 survey than those undertaken in 2015 and 2019, so perhaps these results are more reflective of our clinical leadership than the median professional landscape.2
OPTICAL TREATMENTS FOR MYOPIA
This past year has also brought more knowledge on myopia control interventions. New spectacle lens designs incorporating Diffusion Optics Technology (DOT)3 (Figure 2, opposite) and Cylindrical Annular Refractive Elements (CARE)4 have had publication of one-year randomised controlled trial data. The other newest optical intervention is a novel myopia control soft contact lens with ‘noncoaxial ring-focus’ design, for which six-month clinical trial data shows strong performance.5 In all three cases, longer study durations are needed to accurately compare their efficacy to other interventions, given that the most impressive treatment effects tend to be observed in the first six to 12 months.6
Long-term study outcomes enable a treatment to demonstrate its continued effectiveness and acceptance in older children and teenagers. Sixyear data on the Defocus Incorporated Multiple Segments (DIMS) spectacle lens was revealed this year,7 sitting alongside dual-focus concentric soft contact lenses (MiSight 1 day)8 in equal duration of data. In both cases, the benefits of commencing treatment later – switching children from single vision to the intervention at ages 11–16 years – were demonstrated.
Three-year data for spectacle lenses with highly aspherical lenslets (H.A.L.T. technology) has been published,9 and orthokeratology data from up to 18 years of follow-up in clinical practice has shown robust safety and predictability of treatment.10 The continued growth of the evidence base for optical myopia control treatments provides clinicians and parents with reassurance.
ATROPINE IN MYOPIA CONTROL
Topical atropine treatment has been a fascinating scientific journey to follow, with the past decade seeing the rise of 0.01% as the preferred strategy, after the ATOM2 (Atropine for Treatment Of childhood Myopia) five-year study appeared to indicate 0.01% as having similar efficacy to 0.1% and 0.5%, with far less side effects.11
A closer look at the data, though, showed that the final axial eye lengths were no different between the groups, indicating no significant difference in myopia control effects.12 Also, the protocol had no control group, and included one year of treatment cessation, where a rebound effect (accelerated eye growth) was observed as higher in the stronger concentrations. On retrospect, this made the ATOM2 results difficult to apply to clinical prescribing, but this did not prevent the popularity of 0.01% atropine for myopia control growing worldwide based on this study.
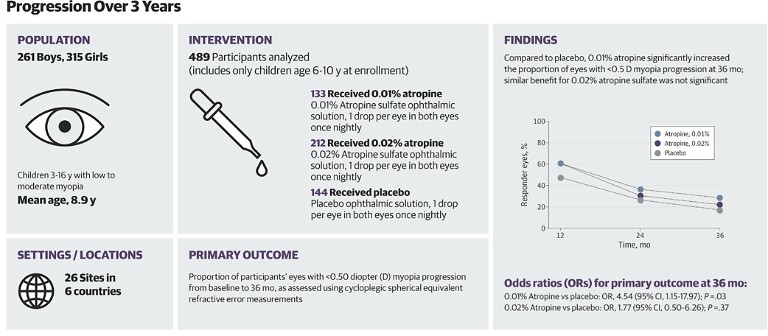
Figure 3. RCT: Efficacy and safety of 0.01% and 0.02% atropine for the treatment of paediatric myopia progression over three years. The key finding is noted in the chart at right, where 28.5% of children treated with 0.01% met the ‘responder’ criteria of exhibiting less than 0.50D myopia progression over three years, compared with 17.5% in the placebo group. 17 Open access.
“ New data has explored combination treatment with mixed results ”
The newer LAMP (Low-Concentration Atropine for Myopia Progression) study revealed a different story. The one-year data, published in 2019, was the first to show a dose response of topical atropine where 0.05% was more effective than 0.025%, which was more effective than 0.01%, when all were compared with placebo treatment. After three years, 0.05% was confirmed as the most effective concentration of the three, with 0.025% and 0.01% showing minimal difference in treatment effect after the first year.13
NEW HORIZONS IN ATROPINE TREATMENT
The latest atropine research has explored commercially prepared formulations of 0.01%, aiming to create stable and consistent treatment compared with the variability found in compounded preparations.14 Just as we understand that different myopia controlling spectacle or contact lens designs have different properties and may have varying treatment effects, the same could be true of topical atropine formulations.
Across the past year, clinical trial data has been reported showing that specific formulations of atropine 0.01% could be beneficial for target populations. A study undertaken in Western Australia showed that efficacy after one year was reasonable for children of ‘European’ or ‘other/mixed’ ancestry, while there was no effect for Australian children of East Asian or South Asian ancestry. The two-year data showed less success, being affected by drop-out rates.15
This ‘target population’ finding agreed with results from a clinical trial in Ireland of a commercially prepared, preservative free 0.01% atropine formulation. The participants were mostly of white European ethnicity, and greater effectiveness was found in blue-eyed compared to green- or brown-eyed children.16
Eye colour was not found to be a predictor of treatment success, though, in two large-scale clinical trials in the US and Europe. The CHAMP study investigated atropine 0.01% and 0.02% over three years, at 26 sites across the US and Europe. In a study population of 54% white ethnicity, modest treatment effects were found in six to 10 year olds treated with 0.01% atropine. Interestingly, 0.02% atropine was not effective, cited as due to a “higher than expected placebo responder rate” (Figure 3).17
Another large, multi-site US study with 45% white ethnicity children found no overall treatment effect, with no benefits found in sub-groups based on age, sex, ethnicity eye colour or baseline refraction.18 Further analysis of the data from Ireland16 and CHAMP (US and Europe) study17 is to come, which should translate into clinical guidance on suitability for this specific atropine product, which is not yet commercially available.
One intriguing study from Singapore recently examined 0.01% topical atropine in comparison with 0.0025% and 0.005%. These super low concentrations represent one-quarter and one-half of the concentration of 0.01% respectively. A better treatment outcome of 0.01% was noted here than in the LAMP study (undertaken on a similar, primarily ethnic Chinese population), with 0.005% and 0.01% appearing statistically similar. No treatment effect was found with the 0.0025% concentration, compared with placebo treatment.19 You can read more about atropine on page 63 of this issue of mivision.
COMBINING ATROPINE WITH OPTICAL TREATMENTS
The ‘sweet spot’ for atropine 0.01% treatment in the myopia control armoury could be its effectiveness when combined with optical treatments. There is a reasonable evidence base now for the benefit of combining atropine 0.01% with orthokeratology treatment to ‘boost’ efficacy, although the treatment ‘boost’ appears to wane after around six months.20,21 Clinically, this means that we may elect to add atropine at initiation of orthokeratology treatment in fast progressors – where the best predictor of this is current age. Children up to 12 years of age tend to show the fastest myopia progression, particularly those up to age 10.22,23
“ Eye colour was not found to be a predictor of treatment success ”
New data has explored combination treatment with mixed results. Atropine 0.01% is not effective in ‘boosting’ the efficacy of centre-distance multifocal monthly (+2.50 Add)24 or dual-focus daily25 soft contact lenses. There may be a benefit in adding atropine to DIMS spectacle lenses, but this data comes from clinical observations without matched treatment groups.26 Other spectacle or contact lens interventions have not yet been investigated for their combination effectiveness with 0.01% atropine.
A GREEN LIGHT FOR RED LIGHT THERAPY?
Repeated low-level red light (RLRL) therapy is the newest myopia control treatment making waves in the landscape. The source of these waves is the combination of highly impressive treatment effects found in one-year27 and two-year28 clinical trial data, with concerning rebound effects on treatment cessation28 and potential ocular health impacts.29
The theory of RLRL therapy is to create a timeeffective alternative to increasing bright light exposure in children for myopia control. The 650nm emitting instrument has reportedly been used for amblyopia treatment in China. The treatment protocol involves using a desktop light therapy device (by Eyerising International) under parental supervision for no more than three minutes, twice per day with a break of at least four hours between treatments, five days per week. The web-linked system tracks compliance and limits treatment time to the specific protocol. Figure 4 shows the instrument in action. You can read more about Eyerising’s RLRL technology on page 45 of this issue of mivision.
In the first published clinical trial, Chinese children aged eight to 13 years with -1.00 to -5.00D of myopia, astigmatism of 2.50D or less, anisometropia of 1.50D or less, and bestcorrected visual acuity (BCVA) of at least 6/6 were all fitted with single vision spectacles. Half were randomised to receive the RLRL treatment. Almost 250 children completed 12 months of treatment, with the effects being a notable 66% axial length and 75% refractive control effect (0.26mm and 0.59D less myopia progression respectively).27 In the second year, the axial length control effect was just over 50%.28 Most remarkable was the finding that at 12 months, around 20% of the children showed axial length stability or shortening of the measurement. Changes in choroidal thickness, indicating increased retinal blood flow, did not fully account for this shortening, indicating some other mechanism is at play.28
Figure 4. The RLRL device in action.27 Open access.
“ The theory of RLRL therapy is to create a time-effective alternative to increasing bright light exposure in children for myopia control ”
These unique findings in a myopia control study have been challenged by the rebound effects found in the two-year data, where children who ceased treatment after one year showed significantly faster eye growth in the second year of 0.42mm, compared with 0.28mm in the single vision corrected, no-treatment group.28 While the clinical trial reported no adverse visual or ocular health outcomes, a single case study was published in May 2023 indicating retinal damage sustained in a 12-year-old Chinese girl. After five months of RLRL treatment, she reported prolonged afterimages and blurred vision which presented as 6/9 in both eyes. No ocular or systemic inflammation was noted, with specific retinal imaging findings recovering and acuity returning to 6/7.5 in both eyes after three months of treatment cessation. The authors stated that solar and laser pointer retinal injuries present similarly, but the patient denied any such exposure.29
At this stage, the level of concern regarding safety is challenging to determine, when pitching this troubling case study of one patient against clinical trial data outcomes on hundreds of patients.
Indeed, another study in China comparing the RLRL device with a “sham device with only 10% of the original device’s power” confirmed impressive myopia control effects of 0.02mm mean axial length increase in six months for the 100% RLRL device compared with 0.13mm for the sham device.30 Six-month data does demand conservative application but generally tends to be indicative of ongoing effects.6 More clinical trials are underway, including at the Australian College of Optometry,31 as well as in the US and Japan in both paediatric and adult populations.32
MUCH LEARNT AND MORE TO COME
The past year been witness to innovations in new myopia control treatments, publication of foundational data confirming long-term safety and efficacy of currently available treatments, and further understanding on the impacts and approaches to clinical myopia management.
It is an exciting time to be keeping an eye on this cutting-edge science – and putting it into practice for the benefit of our young patients with myopia.
Dr Kate Gifford PhD BAppSc(Optom)Hons FAAO is a clinician-scientist and peer educator in private practice in Brisbane. She holds four professional fellowships, has written over 80 peer reviewed and professional publications, and has presented over 160 conference lectures around the world, primarily on clinical myopia management. Dr Gifford is the Chair of the Clinical Management Guidelines Committee of the International Myopia Institute and lead author on its report. She is a co-founder and director of the education platform Myopia Profile.
References available at mivision.com.au