mieyecare
What the Hole?!
When to Refer Retinal Holes or Tears
WRITERS Nicole Lawson and Dr Mitchell Lee
It is that all too familiar scenario; it’s Friday afternoon and a patient presents with suddenonset flashes and floaters. After confirming the patient’s history and symptoms to identify potential risk factors, a careful and detailed dilated fundus examination is required to elicit potential underlying causes of the presenting symptoms.
In this article, Nicole Lawson from the Centre for Eye Health (CFEH) discusses when it is appropriate to refer retinal holes, tears, and considerations for prophylactic treatment.
When examining a patient in the circumstances as outlined, it is critical to identify any peripheral retinal lesions to ensure appropriate patient management and reduce the risk of potential vision loss. Up to 50% of symptomatic retinal breaks with associated vitreoretinal traction will develop a retinal detachment.1-3 Understanding the underlying factors influencing the risk of developing a retinal detachment may help guide the clinician regarding the urgency of referral and appropriate patient management.
RISK FACTORS FOR RETINAL TEAR OR DETACHMENT
The decision to refer a patient with a retinal hole and the urgency of such referral is influenced by the risk of progression to retinal detachment. A posterior vitreous detachment (PVD) involves the separation of the posterior vitreous cortex from the internal limiting membrane of the retina and can be associated with vitreous traction. This vitreous traction may lead to mechanical forces on the retina, particularly at the location of vitreoretinal interface abnormalities, which can then lead to the development of retinal breaks.
The rate of developing a retinal tear at presentation following a symptomatic PVD varies according to the literature, but is between 5–27%.2,4 Approximately 1–3% of patients will develop delayed retinal pathology after an initial PVD with a recent large-scale retrospective study by Vangipuram et al. reporting that 2.42% of patients developed a delayed retinal break or detachment up to one year after the initial PVD.5 The authors also concluded that the presence of vitreous haemorrhage, history of retinal break or detachment in the fellow eye, presence of lattice degeneration, or myopia, increased the risk of developing delayed retinal pathology following a PVD.
Additionally, history of trauma or previous intraocular surgery may increase the patient’s risk of developing a retinal tear or detachment.6
CLINICAL EXAMINATION
A detailed and thorough dilated fundus examination is crucial to identifying lesions in the peripheral retina, particularly in a patient with sudden-onset symptoms. The use of a three-mirror lens or indirect ophthalmoscope with scleral indentation allows the clinician to more accurately assess the peripheral retina.
Following dilation, assessment of the anterior vitreous is also required to identify the presence of pigment cells within the anterior vitreous (tobacco dust or Shafer’s sign). These are believed to be from liberated retinal pigment epithelial (RPE) cells and are associated with a high risk of a retinal break.7,8 In the absence of prior ocular surgery, presence of these cells indicates a retinal break until proven otherwise. In patients who have had previous intraocular surgery, this sign is less specific for a new retinal tear and may instead be a result of surgery.
Cells are best viewed with a bright, narrow slit lamp beam aimed posterior to the crystalline lens to illuminate the anterior vitreous cavity. Much like when assessing for cells in the anterior chamber, patient eye movement may help visualisation. It is important to note that the presence of red cells within the anterior vitreous indicates a vitreous haemorrhage, and therefore an increased risk of developing a retinal break or detachment.
DIAGNOSTIC IMAGING
Although not a substitute for dilated fundus examination, advances in imaging technologies, such as ultra-widefield (UWF) imaging and peripheral optical coherence tomography (OCT), help aid the clinician in the detection and management of peripheral retinal pathology.9
The Optos Optomap, and more recently, the ZEISS Clarus UWF imaging devices have shown varying levels of sensitivity and specificity across the literature in identifying peripheral retinal pathology.10-12 In cases where retinal lesions were located anterior to the equator, UWF imaging showed poorer performance compared to lesions posterior to the equator.13 Additionally, retinal lesions located inferiorly and superiorly were more likely to be missed by UWF imaging.10 This may be attributed to difficulties in imaging these far peripheral locations due to patient positioning, lid and eyelash artefacts, as well as media opacities. Treatment-requiring peripheral retinal lesions were also more likely to be missed in UWF imaging in pseudophakic patients,10 possibly due to the presence of posterior capsular opacification or optical aberrations of the intraocular lens when viewing peripherally. UWF directed peripheral steering may be utilised on both the Optomap and Clarus devices to help locate more peripheral retinal lesions, however a recently published study by Jung et al. showed approximately 9% of peripheral retinal breaks may still be missed with Optos peripheral steering.14
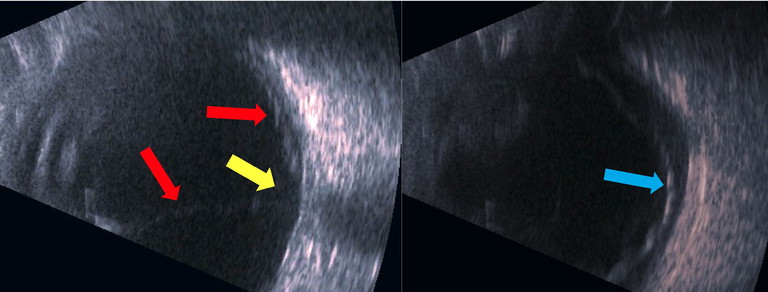
Figure 1: B-scan ultrasonography showing a macula-off retinal detachment (red and blue arrows) in a 55-year-old patient who presented to the CFEH with a white cataract where no fundus view could be obtained. Note the distinctive funnel configuration of the retinal detachment (red arrows) with attachment at the optic nerve (yellow arrow). These images show the retinal detachment, observed on various angles with B-scan ultrasonography.
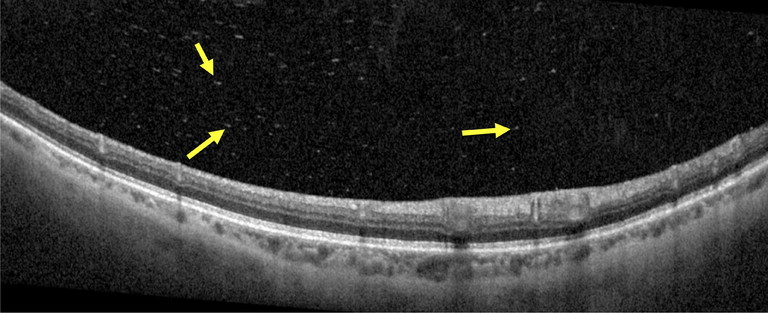
Figure 2. High resolution spectral domain OCT image demonstrating the presence of posterior vitreous opacities (yellow arrows) in an acute PVD associated with vitreous haemorrhage.
B-scan ultrasonography may be required in cases of large vitreous haemorrhage obscuring the view of the retina, or significant media opacity (Figure 1).
The use of peripheral OCT imaging, such as Spectralis OCT (Heidelberg Engineering), may help the clinician identify and more accurately diagnose underlying peripheral retinal pathology. The clinician’s decision to refer or monitor may also be aided by the use of peripheral OCT imaging. In the case of an operculated hole confirmed with OCT imaging, the clinician may be more inclined to monitor the patient. Conversely, a retinal hole associated with areas of vitreoretinal traction and underlying subretinal fluid may require referral for further management.
More recently, the Optos Silverstone uses single-capture UWF imaging with integrated swept-source OCT to capture fundus images and widefield OCT scans. OCT imaging can be guided to a specific retinal lesion located on the central 200° image to assist in diagnosis.15,16 Unfortunately, OCT imaging with the Silverstone cannot be used with peripheral steering, limiting the use of the device to lesions within the central 200° image.
High resolution OCT imaging aids in diagnosing a PVD with separation of the posterior vitreous face from the retina being observed at the macula and the optic disc. The use of OCT imaging may also help identify the presence of posterior vitreous opacities (PVO), believed to be either liberated RPE cells or erythrocytes associated with vitreous haemorrhage. Aishwarya et al. reported a statistically significant relationship between PVO observed on OCT (Figure 2) and the presence of a retinal tear, with 87% specificity and 73% sensitivity.17
In the future, the further development of deep learning-based artificial intelligence may be utilised to assist in screening patients for the identification of retinal breaks and/or detachments with UWF imaging, particularly in a public ophthalmology setting.18
TYPES OF RETINAL HOLES OR TEARS
Atrophic Retinal Holes
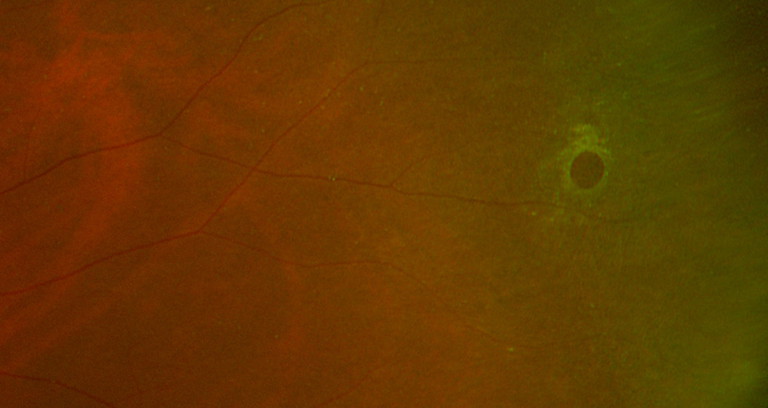
These are full-thickness retinal breaks, typically circular in shape (Figure 3), traditionally believed to be due to retinal thinning or degeneration and unrelated to vitreoretinal traction. They may be associated with a fluid cuff, or in the case of a chronicity, surrounded by RPE hyperplasia. They may also occur within lattice degeneration. More recent research has shown vitreous adhesion and traction associated with atrophic retinal holes, with similar morphology to macular holes.19 The incidence of developing a retinal detachment from atrophic holes associated with lattice degeneration is less than 2%,20 with often shallow, slow progressing and asymptomatic detachments.21,22
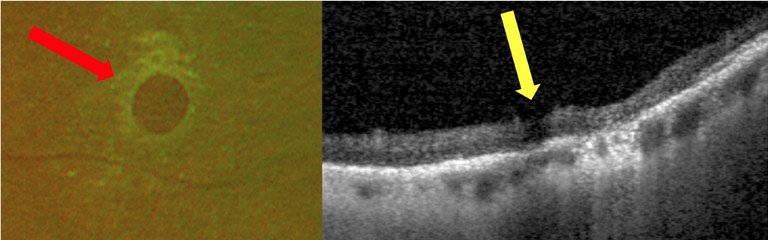
Figure 3. Examples of an atrophic retinal hole observed on Optos UWF imaging (red arrow) and OCT peripheral imaging (yellow arrow).
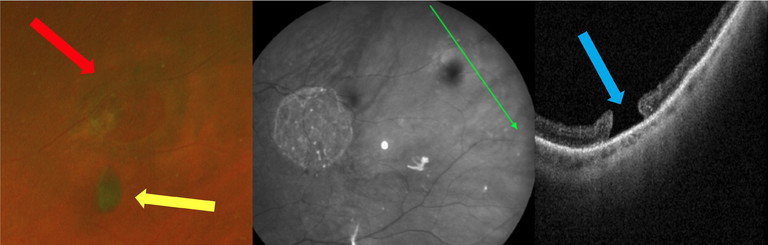
Figure 4: An operculated retinal hole found in an asymptomatic 66-year-old patient who presented to CFEH. UWF imaging showing the underlying retinal hole (red arrow) and free-floating overlying operculum (yellow arrow). Peripheral OCT imaging confirmed the full-thickness hole (blue arrow) with release of previous vitreoretinal traction.
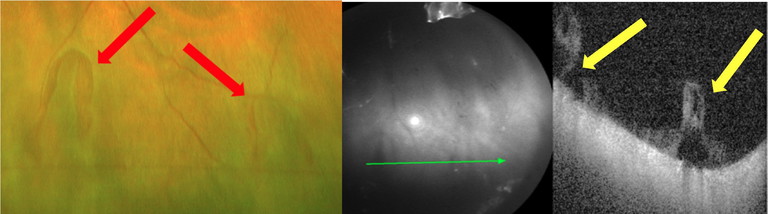
Figure 5. Two horseshoe tears observed in a 65-year-old symptomatic patient with a history of an acute posterior vitreous detachment six months prior. The UWF image shows two separate retinal tears (red arrows) with peripheral OCT confirming the retinal breaks and persistent vitreoretinal traction (yellow arrows).
Although prophylactic laser retinopexy of atrophic retinal holes may reduce the risk of retinal detachment, the literature remains limited.23 The American Academy of Ophthalmology (AAO) Preferred Practice Pattern (PPP) guidelines suggest that “asymptomatic atrophic retinal breaks rarely need treatment”.6 Furthermore, according to the PPP, if an atrophic hole associated with lattice degeneration has “minimal subretinal fluid without progression, or lacks evidence of a PVD, it does not require treatment”.
Operculated Retinal Holes
These are full-thickness retinal breaks caused by localised vitreoretinal traction (Figure 4). The traction causes detachment of a circular ‘plug’ (operculum) of neurosensory retina, observed floating over the retinal hole. Similar to atrophic holes, there may be an associated fluid cuff, or RPE hyperplasia suggesting chronicity. Operculated holes rarely progress to retinal detachment, unless the vitreous remains attached bordering the hole.1
Routine monitoring of operculated holes is recommended with peripheral OCT imaging to assist in initial diagnosis and monitoring.
Horseshoe Tears
These are full-thickness U-shaped breaks with similar pathophysiology to operculated holes (Figure 5), and are often associated with an acute posterior vitreous detachment. The vitreous remains attached to the flap with the ongoing traction increasing the risk for progression to a retinal detachment. The base of the tear typically points anteriorly, while the apex points posteriorly. Risk factors for developing a horseshoe tear include age, myopia, lattice degeneration, trauma, and previous intraocular surgery.24
Prompt referral to an ophthalmologist is required for all horseshoe tears, which should be regarded as very high risk for progression to detachment. There is a higher risk for developing a retinal detachment if the tear is located superiorly compared with inferior.25
Giant Retinal Tear and Retinal Dialysis
Giant retinal tears are full-thickness circumferential breaks extending over at least three clock hours of the retina in the presence of a PVD. The underlying pathophysiology is not fully understood with the majority of cases being idiopathic, however trauma, previous intraocular surgery, high myopia, and inherited vitreoretinopathies, such as Stickler syndrome, have been associated with the condition.26 Prompt referral is required with management posing a challenge given the large area of detachment.27 Surgery in these cases requires the use of heavy liquids to unfold the detached retina. Silicone oil tamponade with strict positioning is important to ensure adequate retinopexy and reduce the risk of retinal slippage. There is a higher risk of the fellow eye developing retinal pathology with routine careful dilated fundus examinations being required.28
Retinal dialysis is a full-thickness break that occurs at the ora serrata. It is typically associated with trauma and predominantly affects young males.29 A posterior vitreous detachment is present in approximately half of these patients.30 There is a high risk of developing a retinal detachment and prompt referral is required. These cases often present insidiously, with retinal detachment several weeks or months after blunt trauma. The dialysis itself is located in the far periphery and often visible only with indented examination. Associated detachments tend to be shallow, slowly progressing, and respond extremely well to scleral buckle surgery.
“Prompt referral to an ophthalmologist is required for all horseshoe tears, which should be regarded as very high risk for progression to detachment”
SUMMARY
For patients presenting with sudden-onset flashes and floaters, prompt and careful dilated examination is required to identify underlying treatment requiring retinal pathology. The use of diagnostic imaging may aid the clinician in the appropriate diagnosis and management. Incidentally found lattice degeneration, atrophic holes, and operculated holes can typically be monitored, with careful patient counselling regarding the symptoms of a retinal tear or break. A lower threshold for referral is wise where there are symptoms of flashes or floaters, evidence of traction or subretinal fluid at the lesion, and/or development of new breaks in eyes that have previously undergone a vitrectomy. The presence of vitreous haemorrhage should also require prompt referral, as the chance of a retinal break or other serious pathology is much higher in these cases. Literature guiding evidence-based practice regarding the use of prophylactic treatment of these lesions remains limited. Horseshoe tears, giant retinal tears and retinal dialysis require prompt same-day referral to a vitreoretinal specialist for appropriate timely management to reduce the risk of vision loss.
Nicole Lawson Bsc MClinOptom is a staff optometrist at the Centre for Eye Health in Sydney. Ms Lawson completed her Bachelor of Optometry and Bachelor of Science (Vis Sci) at the University of New South Wales.
She has worked in full scope practice since graduating, with particular interest in early detection and intervention of ocular pathology. She is also a clinical optometrist supervisor at the UNSW School of Optometry.
Dr Mitchell Lee BSc MBBS (Hons) MMed(Critical Care) FRANZCO is an ophthalmologist who specialises in vitreoretinal surgery, as well as complex anterior segment, cataract, and lens surgery. He is a Director at Eagle Eyes Surgeons in Mosman, Sydney.
References
1. Davis MD. The natural history of retinal breaks without detachment. Trans Am Ophthalmol Soc. 1973;71:343-372.
2. Shea M, Davis MD, Kamel I. Retinal breaks without detachment, treated and untreated. Mod Probl Ophthalmol. 1974;12(0):97-102.
3. Ghazi NG, Green WR. Pathology and pathogenesis of retinal detachment. Eye (Lond). 2002;16(4):411-421. doi: 10.1038/sj.eye.6700197.
4. Seider MI, Conell C, Melles RB. Complications of acute posterior vitreous detachment. Ophthalmology. 2022;129(1):67-72. doi: 10.1016/j.ophtha.2021.07.020.
5. Vangipuram G, Li C, Li S, et al. Timing of delayed retinal pathology in patients presenting with acute posterior vitreous detachment in the IRIS® registry (Intelligent Research in Sight). Ophthalmol Retina. 2023;7(8):713-720. doi: 10.1016/j.oret.2023.04.004.
6. Flaxel CJ, Adelman RA, Bailey ST, et al. posterior vitreous detachment, retinal breaks, and lattice degeneration Preferred Practice Pattern® [published correction appears in Ophthalmology. 2020 Sep;127(9):1279. doi: 10.1016/j.ophtha.2020.06.049]. Ophthalmology. 2020;127(1):P146-P181. doi: 10.1016/j. ophtha.2019.09.027.
7. Tanner V, Harle D, Tan J, Foote B, Williamson TH, Chignell AH. Acute posterior vitreous detachment: the predictive value of vitreous pigment and symptomatology. Br J Ophthalmol. 2000;84(11):1264-1268. doi: 10.1136/ bjo.84.11.1264.
8. Gishti O, van den Nieuwenhof R, Verhoekx J, van Overdam K. Symptoms related to posterior vitreous detachment and the risk of developing retinal tears: A systematic review. Acta Ophthalmol. 2019;97(4):347-352. doi: 10.1111/aos.14012.
9. Cheung R, Ly A, Katalinic P, et al. Visualisation of peripheral retinal degenerations and anomalies with ocular imaging. Semin Ophthalmol. 2022;37(5):554-582. doi: 10.1080/08820538.2022.2039222.
10. Khan M, Kovacs K, Guan I, et al. evaluating ultrawidefield imaging utility in the detection of treatmentrequiring peripheral retinal tears and holes. Retina. 2024;44(1):71-77. doi: 10.1097/IAE.0000000000003918.
11. Karatepe Hashas AS, Popovic Z, Abu-Ishkheidem E, et al. A new diagnostic method for retinal breaks in patients with posterior vitreous detachment: Ultra-widefield imaging with the Zeiss Clarus 700. Acta Ophthalmol. 2023;101(6):627-635. doi: 10.1111/aos.15652.
12. Yang D, Li M, Wei R, Xu Y, Shang J, Zhou X. Optomap ultrawide field imaging for detecting peripheral retinal lesions in 1725 high myopic eyes before implantable collamer lens surgery. Clin Exp Ophthalmol. 2020;48(7):895-902. doi: 10.1111/ceo.13809.
13. Mackenzie PJ, Russell M, Maberley DA, et al. Sensitivity and specificity of the Optos optomap for detecting peripheral retinal lesions. Retina. 2007;27(8):1119-1124. doi: 10.1097/IAE.0b013e3180592b5c.
14. Jung JJ, Lim SY, Chan X, et al. Ultra-widefield imaging detection rate in identifying peripheral retinal tears in single versus montage of peripheral steering. Retina. 2024;44(3):406-413. doi: 10.1097/IAE.0000000000003979.
15. Kovacs KD, Mahrous MA, Gonzalez L, et al. Feasibility and clinical utility of ultra-widefield-navigated swept-source optical coherence tomography imaging. J Vitreoretin Dis. 2021;5(5):396-404. doi: 10.1177/2474126421997335.
16. Kim J, Choi KS. peripheral lattice degeneration imaging with ultra-widefield swept-source optical coherence tomography. Korean J Ophthalmol. 2023;37(6):485-489. doi: 10.3341/kjo.2023.0074.
17. Rao AV, Shah AR, Nguyen VT, et al. Use of optical coherence tomography in detecting retinal tears in acute, symptomatic posterior vitreous detachment. Retina. 2023;43(5):802-807. doi: 10.1097/IAE.0000000000003718.
18. Christ M, Habra O, Monnin K, et al. Deep learningbased automated detection of retinal breaks and detachments on fundus photography. Transl Vis Sci Technol. 2024;13(4):1. doi: 10.1167/tvst.13.4.1.
19. Govetto A, Sebag J, Lucchini S, et al. Imaging rhegmatogenous retinal lesions and peripheral vitreoretinal interface with widefield optical coherence tomography. Retina. 2024;44(2):269-279. doi: 10.1097/ IAE.0000000000003946.
20. Sasaki K, Ideta H, Oka C, et al. Risk of retinal detachment in patients with lattice degeneration. Jpn J Ophthalmol. 1998;42(4):308-313. doi: 10.1016/s00215155(98)00012-4.
21. Blindbaek S, Grauslund J. Prophylactic treatment of retinal breaks – a systematic review. Acta Ophthalmol. 2015;93(1):3-8. doi: 10.1111/aos.12447.
22. Murakami-Nagasako F, Ohba N. Phakic retinal detachment associated with atrophic hole of lattice degeneration of the retina. Graefes Arch Clin Exp Ophthalmol. 1983;220(4):175-178. doi: 10.1007/BF02186664.
23. Wilkinson CP. Interventions for asymptomatic retinal breaks and lattice degeneration for preventing retinal detachment. Cochrane Database Syst Rev. 2014;2014(9):CD003170. doi: 10.1002/14651858. CD003170.pub4.
24. Peng ET, Parvus MN, Yu HJ, et al. Risk factors for the development of fellow eye horseshoe retinal tears following horseshoe retinal tear in the presenting eye. Ophthalmic Surg Lasers Imaging Retina. 2023;54(6):338345. doi: 10.3928/23258160-20230523-01.
25. Garoon RB, Smiddy WE, Flynn HW Jr. Treated retinal breaks: Clinical course and outcomes. Graefes Arch Clin Exp Ophthalmol. 2018;256(6):1053-1057. doi: 10.1007/s00417-018-3950-8.
26. Ramamurthy S, Raval V, Ali H, et al. giant retinal tear detachment: Clinical presentation and treatment outcomes in 396 patients. Retina. 2023;43(5):784-792. doi: 10.1097/ IAE.0000000000003720.
27. Bleicher I, Miller JB. Giant Retinal Tears: A Review With a Focus on Trauma. Int Ophthalmol Clin. 2024;64(2):107-123. doi:10.1097/IIO.0000000000000491.
28. Sengillo JD, Smiddy WE, Yannuzzi NA, Flynn HW Jr; Giant retinal tear study group. giant retinal tears: Long-term outcomes of fellow eyes. Ophthalmic Surg Lasers Imaging Retina. 2022;53(11):619-625. doi: 10.3928/2325816020221018-01.
29. Rohowetz LJ, Jabbehdari S, Smiddy WE, et al. retinal detachment associated with retinal dialysis: Clinical features and outcomes of surgery in a 10-year study. Ophthalmol Retina. 2023;7(10):857-861. doi: 10.1016/j. oret.2023.06.013.
30. Mitry D, Singh J, Yorston D, et al. The predisposing pathology and clinical characteristics in the Scottish retinal detachment study. Ophthalmology. 2011;118(7):14291434. doi: 10.1016/j.ophtha.2010.11.031.