mieducation
Drought Breaker: New GA Treatments to Impact Optometry
One of the defining moments in ophthalmology was the introduction of intravitreal anti-VEGFs to treat neovascular age-related macular degeneration (nAMD). Now, eye care professionals outside the United States await news from their respective regulatory bodies about the approval of the newest intravitreal injection (IVI) agents, pegcetacoplan and avacincaptad pegol. Given the green light by the United States Food and Drug Administration (FDA) last year, these drugs represent the first-ever treatments for geographic atrophy (GA) – and the first real hope for clinicians and patients alike.
So, what would a post-approval patient pathway look like, and how can we manage their expectations?
WRITERS Dr Devinder Chauhan and Dr Alexander Tan
LEARNING OBJECTIVES
On completion of this CPD activity, participants should be able to:
1. Be aware of the GA treatment options awaiting TGA approval,
2. Understand the pathology, symptoms, and functional impact of GA,
3. Be able to identify GA using OCT,
4. Describe an ideal management pathway for patients with GA, and
5. Realise the role of optometry in screening, referring on, counselling, and maintaining communication between patients with GA and retinal specialists.
AGE-RELATED MACULAR DEGENERATION: NOW AND THEN
AMD, a primary cause of irreversible vision loss in older populations, places a substantial socioeconomic burden on both individual sufferers and societies at large. 1
This progressive, degenerative retinal disease develops over decades and has two main advanced forms – GA and nAMD (also known as ‘wet’ AMD) – which together affect approximately 10% of people with AMD.
Globally, AMD is among the leading causes of blindness in adults aged 50 years and over. GA accounts for 20% of all legal blindness attributed to AMD 2,3 and affects more than five million people worldwide. Genetics account for 70% of the risk for progressive AMD, including GA. After the age of 50, the prevalence of GA approximately quadruples every 10 years, and around 75,000 Australians have GA in at least one eye.
A concerning aspect of AMD management is the rate of diagnosis – or rather the lack thereof – particularly for the ‘dry’ forms. Several factors contribute to this problem, including the asymptomatic nature of early AMD, an acceptance of slightly poor vision by elderly patients as ‘just another part of ageing’, and previously limited imaging and analysis options in optometry practice. Historically, the latter has not been a concern because there has not been a treatment available for GA. Now, the increasing availability of advanced retinal imaging technologies has resulted in community optometrists possessing powerful tools to identify and monitor the earliest signs of AMD.
DISEASE PROGRESSION
Understanding the natural history of AMD is pivotal to its ideal management. This disorder doesn’t manifest overnight – rather, it progresses through a spectrum, spanning early to late stages, over decades.
Early Age-Related Macular Degeneration At this point, patients may have minimal or no symptoms. Diagnosis is usually made by the presence of small- to medium-sized drusen – yellowish deposits located between the retina and the choroid. These consist predominantly of lipofuscin, a by-product of cellular activity, and their appearance raises the spectre of AMD progression. 4
Intermediate Age-Related Macular Degeneration Progression to this stage may or may not be associated with visual symptoms. What does change, however, is the drusen size and number. Large drusen become evident, as well as becoming confluent, and pigmentary changes in the retina (due to hypertrophy of the retinal pigment epithelium (RPE)) can be seen on fundoscopy or fundus photography. These are indicative of more advanced cellular damage and denote an increased risk of progressing to late AMD.
Late Age-Related Macular Degeneration The advanced stage of AMD can manifest in two forms (nAMD and GA), and patients may have one or both in the same eye.
nAMD is characterised by the rapid onset of vision loss due to abnormal blood vessel growth from the choroid towards and under the retina. As its name implies, this form is ‘wet’ due to the invasion of abnormal blood vessels beneath the retina. These vessels are prone to leakage and bleeding, causing scarring and precipitous vision loss. 5 The abrupt vision deterioration associated with untreated or sub-optimally managed nAMD can precipitate a rapid decline in quality of life, further elevating indirect costs from loss of productivity, increased accidents, and augmented care dependency. 6,7
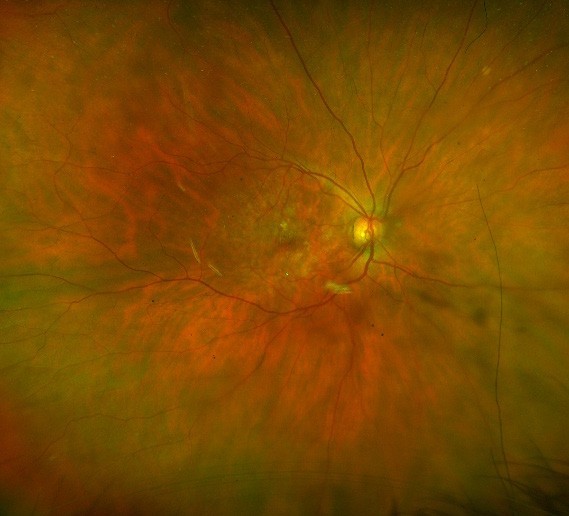
A
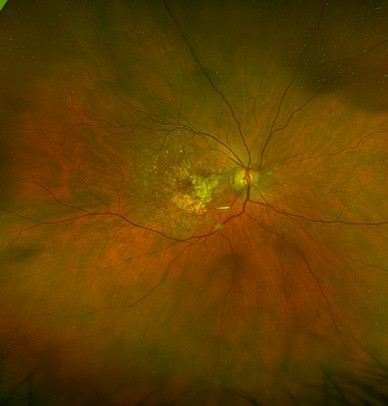
B
Figure 1. Fundus photos demonstrating: A. early AMD (with small drusen) and B. late AMD (with larger drusen and patches of retinal atrophy).
GA, the dry form of late AMD, is characterised by discrete patches of degeneration of the RPE, photoreceptors, and choriocapillaris in the macula. The result is a progressive loss of central vision. 8 Untreated, the pace of GA progression can vary significantly, remaining relatively stable for years in some and progressing rapidly in others. In many cases, the atrophic patches gradually coalesce, leading to more significant vision loss. The speed and pattern of progression are influenced by numerous factors, including genetics, age, and environmental factors.
Many patients with GA remain asymptomatic until the foveal centre is affected – they are either unaware of, or dismiss early symptoms such as difficulties with reading speed and extremes of lighting. In the latter case, glare may result in patients giving up night driving several years before central vision involvement. This is an example of the false assumption among the ageing population that ‘my vision is obviously going to get worse as I get older’.
THE ROLE OF THE COMPLEMENT SYSTEM
The complement system is a key component of the body’s innate immune arsenal.
Comprising a myriad of proteins circulating in our blood plasma and present on cell surfaces, this system serves as a rapidresponse force against microbial invaders.
When activated, these proteins engage in a domino-effect cascade, resulting in a series of reactions that amplify the response, mark invaders for destruction, and recruit other immune cells. In essence, the cascade acts as an unsung hero, working behind the scenes in countless immune processes, from warding off infections to cleaning up dead cells.
However, when left unchecked or misdirected, the complement system can become a doubleedged sword. Abnormalities in its regulation can induce excessive inflammation and unintended damage to our body’s tissues.
Genetic investigations have identified correlations between AMD and variations in genes encoding several complement proteins. The most notable among these is complement factor H (CFH). 9
Under normal conditions, CFH operates as a regulator, curtailing the complement system’s activation to ensure it doesn’t go into overdrive and inflict collateral damage on our own tissues. However, certain genetic mutations can impede the protective function of CFH. This leads to a scenario where the complement system, especially in ocular tissues, remains hyperactive. Such heightened activity in the retinal environment can precipitate chronic inflammation, triggering a cascade of cellular degeneration and death, which are trademarks of GA. 10
With ageing, the RPE is exposed to oxidative stress caused by retinal metabolic demands, photo-oxidation, and environmental stressors. Damage caused by these stressors can accumulate, resulting in formation of extracellular drusen. Excessive drusen accumulation may trigger inflammation via multiple pathways (e.g. the complement cascade), leading to photoreceptor, RPE, and choriocapillaris cell death. 11,12 Loss of photoreceptors, RPE, and choriocapillaris results in sharply defined atrophic lesions, which are characteristic of GA. 11
“ GA accounts for 20% of all legal blindness attributed to AMD 2,3 and affects more than five million people worldwide ”
ASSESSING OCT SCANS FOR GA
While a comprehensive fundoscopic clinical assessment has a very good chance of picking up GA, it can be very difficult to diagnose extrafoveal GA without any symptoms, and without imaging.
In retinal practice, the diagnosis of GA is primarily made using either fundus autofluorescence (FAF) imaging or optical coherence tomography (OCT), or both. The former highlights the absence of RPE, but the latter gives much more insight into the differential loss of layers within the macula.
On OCT, GA lesions are identified by: 13,14
• Loss of RPE and photoreceptor layers,
• External limiting membrane absence, and
• Increase in choroidal hypertransmission.
Several factors predict AMD progression to GA: 15
• Reticular pseudodrusen/subretinal drusenoid deposits – pyramidal drusenoid deposits located above the RPE; these may represent a risk factor for the development of late AMD,
• Subsidence ‘sinking’ of the inner nuclear layer (INL) and outer plexiform layer (OPL) of the retina, which appear to sink towards the RPE in the area of outer atrophy; this appears as a hyporeflective wedge, and
• Hyperreflective foci – these appear as discrete, well-circumscribed, punctate lesions equal or greater in reflectivity than RPE.
Factors associated with an increased rate of GA progression include:
• Affected eye: -Larger baseline lesion size, 16,17 -Multifocality, 18,19
-Abnormal FAF pattern: banded, diffuse FAF phenotypes, 17 and
-Nonfoveal location and progression toward periphery; extrafoveal GA lesions progress faster than foveal lesions. 13,19
• Fellow eye: -Bilateral GA, 13,18,20 and -Higher progression rate in fellow eye. 13 GA is classified as follows: 14
• Incomplete outer retina atrophy (iORA): thinning of the outer retina, intact RPE band, no hypertransmission,
• Complete outer retina atrophy (cORA): severe thinning of the outer retina, intact RPE band, intermittent hypertransmission,
• Incomplete RPE and outer retinal atrophy (iRORA): some hypertransmission, the RPE band is present but irregular, photoreceptor degeneration, and
• Complete RPE and outer retinal atrophy (cRORA): homogenous hypertransmission, absence of the RPE, loss of photoreceptors.
TREATMENT OPTIONS
2005 saw the introduction of intravitreal anti-VEGF therapies, which obstruct the signals for growth and leakage of the abnormal vessels in nAMD. 21
But treatment options for GA remained nonexistent – until recently. In the United States at least, there are two intravitreal therapies targeting the complement system, and more in the pipeline, including stem cell and gene therapies. Whether and when these therapies become available in Australia remains to be seen, pending both Therapeutic Goods Administration (TGA) and Pharmaceutical Benefits Advisory Committee (PBAC) approval, but the overall consensus among ophthalmologists seems to be optimistic.
The two FDA-approved drugs are pegcetacoplan (Syfovre, Appellis Pharmaceuticals) and avacincaptad pegol (Izervay, Iveric Bio). These drugs retard the progression of GA, neither arresting nor reversing it. Consequently these intravitreal injections, which are given every four or eight weeks, may well buy patients more time before they lose their central vision.
Although it appears that almost every patient will eventually lose central vision if they live long enough, there are some early indications that the therapeutic effect increases with time, and that the retardation of progression may increase with prolonged therapy. 22,23 It is unknown whether this trend will continue and potentially even halt progression after several years, as the longest follow-up data presented so far is just three years. 23
There are uncertainties in the United States retina community, and globally, about which patients should be treated, when they should be treated, and how to assess the efficacy of treatment, given that the disease is only retarded by these therapies.
These discussions among clinicians and with patients are informed by the fact that there have been early inflammatory complications associated with pegcetacoplan, the first drug approved, which have resulted in severe visual loss for some patients. Although devastating, the current estimate of retinal vasculitis risk is approximately 0.01% and has, so far, only been associated with real-world administration of the first injection. 23,24
Another factor to consider is that none of the pivotal trials have shown a significant visual benefit. Yes, there has been a significant slowing of the growth of GA lesions, but this has not translated to visual benefit. 22 This may be related to the patient selection for these trials – pegcetacoplan, for example, was administered to patients with subfoveal GA, where there was already poor visual acuity. Another factor may be that even for those that were extrafoveal at the start of the trials, the growth retardation was not enough to prevent the fovea – and thus vision – being affected over the time course of the studies. Both drugs are currently being monitored in Phase 4 (real-world observational) studies.
THE KEY TO OPTIMAL AMD MANAGEMENT
Optometrists are in the unique position of being the first line of defence in ophthalmic health. As the primary touchpoint for many ocular concerns, optometrists screen large numbers of people in the community, meaning they are ideally placed to identify those with OCT findings suggestive of AMD.
They are also educators and advocates. With their comprehensive understanding of AMD and its trajectory – from its nascent stages to late manifestations – optometrists can decide whether referral to a retinal specialist or a ‘watch and wait’ approach is warranted.
A patient pathway that involves community management for as long as possible represents the most appropriate use of our healthcare resources (i.e. the areas of eye care that are best served by optometry as opposed to ophthalmology, based on availability, reach and scope of practice).
But success requires a good working relationship and two-way dialogue between optometrists and the retinal specialists they normally refer to. Only then can we be sure that the right patients are getting referred at the right time.
WHEN TO REFER TO A RETINAL SPECIALIST
In the absence of signs suggestive of late AMD, patients can be advised to return to their optometrist for ongoing reviews, coupled with dietary and lifestyle advice.
When the OCT shows evidence of neovascularisation, the decision to refer is usually clear. Many optometrists then counsel patients accordingly – discussing the aim of the referral, potential treatment options, and managing expectations with respect to treatment.
On the other hand, there is still uncertainty for optometrists, and even for some ophthalmologists, about which patients with GA could be treated down the track, when to refer, and how to advise patients.
If GA does develop, there are several factors that determine the need for referral and/or ongoing monitoring.
Simply having GA is not a cause for referral currently, given that the aforementioned treatments are not yet available in Australia. However, having a patient seen by a retinal specialist at least once early on in the disease would allow a baseline to be established, allowing later comparisons to assess the rate of progression. While an image sharing set-up with full functionality would probably mitigate this need, an ongoing dialogue between optometrists and retinal specialists must be established, nevertheless. Regular communication is critical as the referral criteria and approach to treatment will likely evolve for some time following TGA approval, as we gain first-hand experience with local patients.
CASE ONE: PROGRESSION OF GA
Ms Grant,* a 77-year-old woman presented in 2017 with GA that had already caused vision loss in the left eye to the extent that her visual acuity was counting fingers (CF). The right eye exhibited intermediate AMD, with visual acuity 6/7.5. Her optometrist diagnosed GA and referred her to a retinal specialist for ongoing review and to see if any treatment was possible at that time.
By 2018, the vision in Ms Grant’s right eye had decreased to 6/12, as the drusen collapsed into GA (Figures 2 and 4). In 2023, there was now significant central GA with visual acuity 6/36 in the right eye (Figures 3 and 4), while the left eye acuity remained CF.
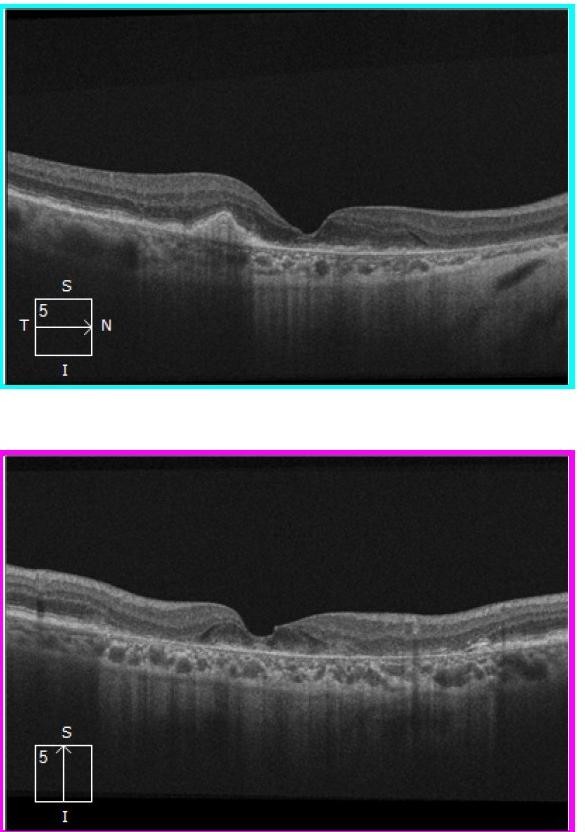
Figure 2. Horizontal and vertical slice OCT images (2018) showing macular atrophy with some preservation of central photoreceptors.
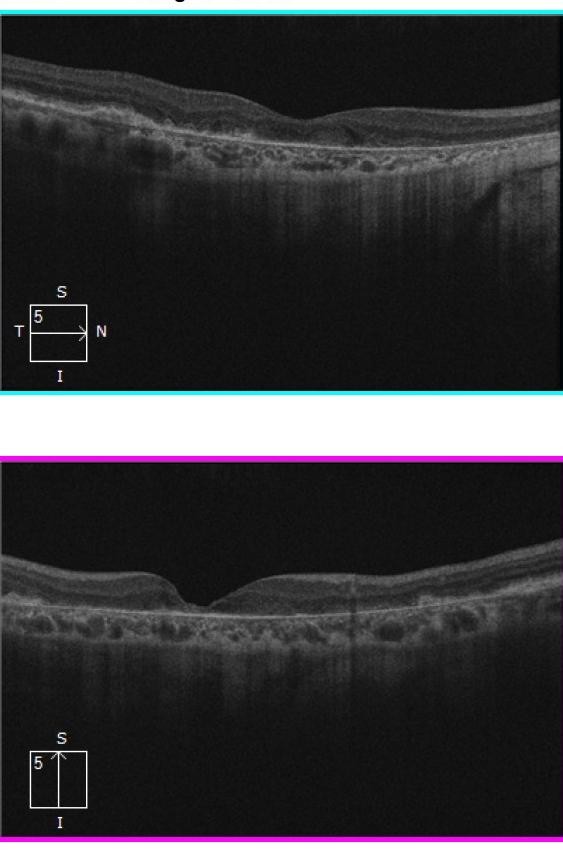
Figure 3. OCT images (2023) showing progressive atrophy with loss of central/foveal photoreceptors.
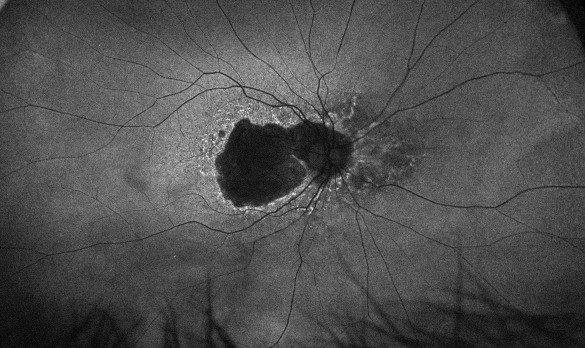
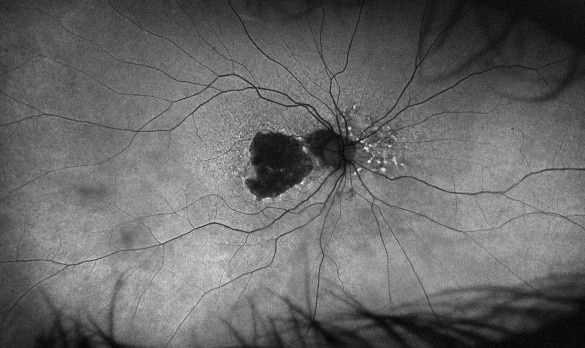
Figure 4. Fundus autofluorescence in 2018 showing significant central atrophy (left), with increasing size of GA and loss of any healthy RPE centrally in 2023 (right).
CASE TWO: CO-EXISTENT nAMD AND GAx
Ms Baker,* a 70-year-old woman presented in November 2016 with nAMD. She had a best corrected visual acuity of 6/24 vision, extensive sub-RPE material, intra- and subretinal fluid, and sub-retinal hyperreflective material (Figure 5).
Serial, monthly intravitreal injections of ranibizumab substantially reduced the subretinal fluid, with some remaining intra-retinal fluid; her pigment epithelial detachment (PED), however, grew significantly (Figure 6) but subsequently flattened. While it may initially be thought of as a positive sign, the natural history of AMD, and sometimes treatment for wet AMD, includes the development of GA in areas of PED collapse.
In this patient’s case, even though total fluid resolution was achieved by July 2017, she had developed subfoveal GA (Figure 7).
The GA was characterised by absent RPE, resulting in white streaks of hypertransmission extending into the choroid. By February 2020, the increased hypertransmission and absent RPE and photoreceptors most of the way across the scan were consistent with extensive sub-foveal GA. Her vision had accordingly dropped dramatically (Figure 8).
Ms Baker’s right eye began with only intermediate AMD in April 2017 (Figure 9), but by October 2023 the OCT (Figure 10) demonstrated several larger hypertransmission areas, with photoreceptor and RPE loss sparing the fovea, indicating GA. The effect of this was that her Snellen visual acuity may have been good (6/7.5), but the peri-foveal changes had a significant deleterious effect on function, reducing her reading speed, ability to recognise faces, and confidence navigating traffic as a pedestrian.
As this case demonstrates, it is possible to have both GA and nAMD in the same eye, which is important to bear in mind. The patient was already under the care of a retinal specialist when signs of GA became evident.
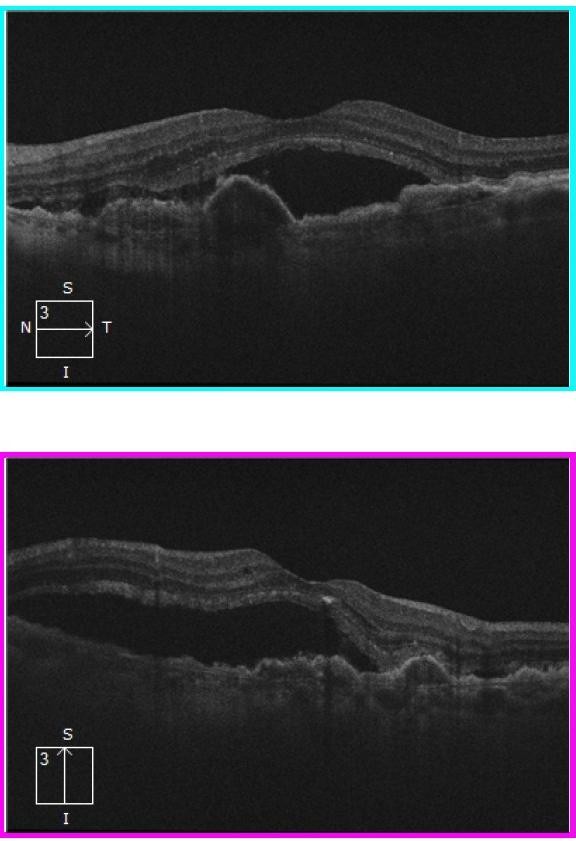
Figure 5. OCT images in left eye showing extensive sub-retinal fluid, PED and confluent drusen.
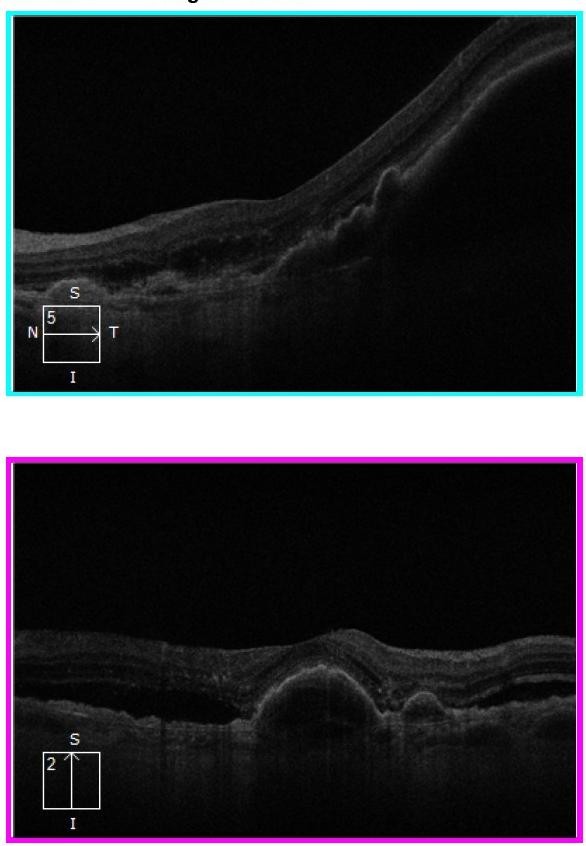
Figure 6. Left eye OCT images: horizontal scan showing a large PED with a sliver of sub-retinal fluid at the centre; vertical scan showing a PED at the fovea, extrafoveal sub-retinal fluid, and some hyperreflective foci superior to the fovea.
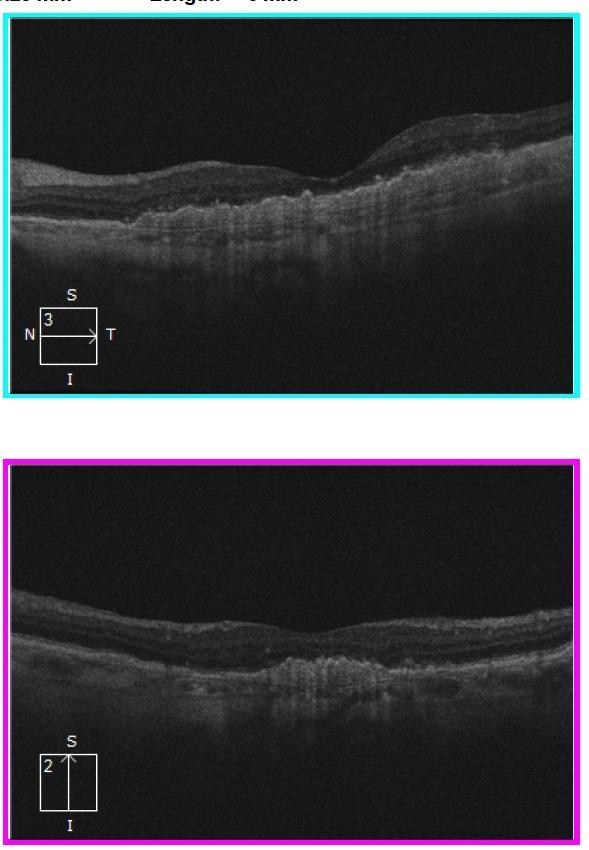
Figure 7. Corresponding left eye OCT images showing persistent PED, albeit smaller and without any sub-retinal fluid present.
CONCLUSION
Given the irreversible nature of GA and the lack of a definitive cure, timely referral to a retinal specialist will be crucial if treatments become available here in Australia. In the meantime, it is prudent to begin identifying patients in preparation for referral for treatment, ensuring the earliest possible intervention and opportunity for greatest therapeutic effect.
*Patient names changed for anonymity.
To earn your CPD hours from this article, visit mieducation.com/drought-breaker-new-gatreatments-to-impact-optometry.
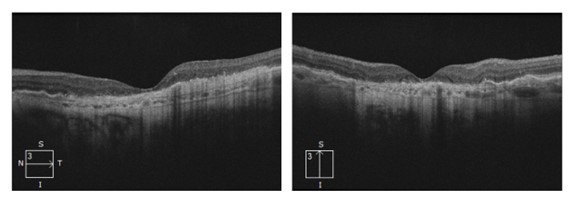
Figure 8. Left eye OCT scans in 2020 demonstrating flattening of the PEDs and drusen associated with extensive GA. The latter is most obvious because of hypertransmission (streaky, high signal, extending downwards from the RPE on both scans).
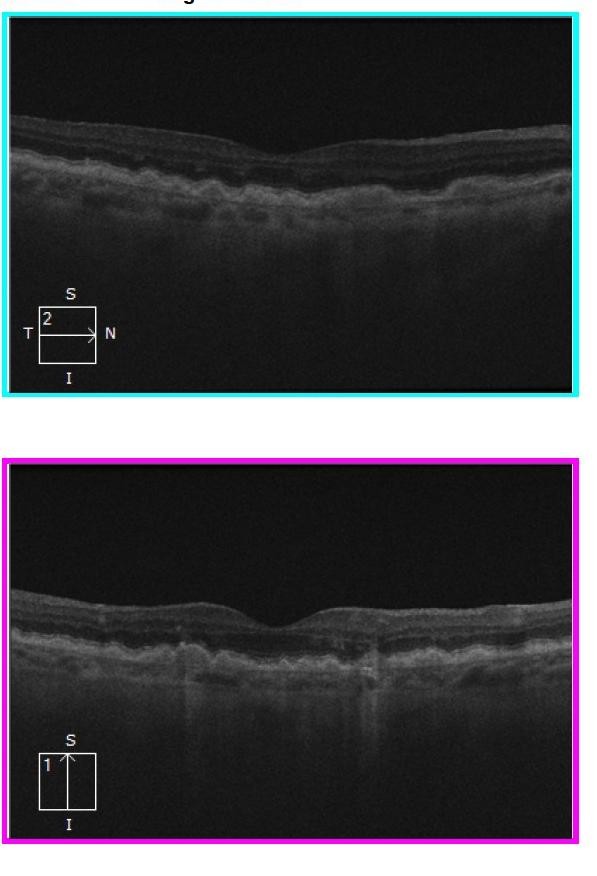
Figure 9. Right eye OCT images demonstrating some areas of interruption of continuity of RPE and associated hypertransmission only in the latter. Both eyes have numerous drusen.
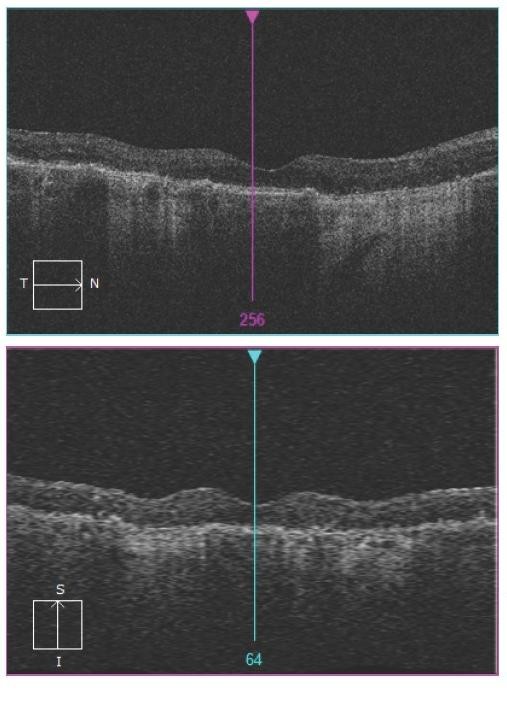
Figure 10. Right eye OCT images with more extensive areas of GA, characterised by thinning and loss of RPE layer and hypertransmission. Note sparing of the centre of the fovea.

Dr Devinder Chauhan MBBS MD FRCOPHTH FRANZCO is a retinal ophthalmologist with over 20 years of experience. Dr Chauhan treats all retinal and macular conditions including macular degeneration and is heavily involved in research in the area of dry age-related macular degeneration. He consults at Vision Eye Institute's clinics in Boronia and Box Hill in Melbourne.

Dr Alexander Tan BMedSci MBBS FRANZCO is a highly skilled comprehensive general ophthalmologist with a special interest in medical retinal disease. He holds public appointments at Monash Health and Eastern Health and consults privately at Vision Eye Institute’s Boronia clinic in Melbourne.
References
1. Wong, W.L., Su, X., Li, X., et al., Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014; 2(2):e106–16.
2. Biarnés, M., Monés, J., Arias, L., Update on geographic atrophy in age-related macular degeneration. Optom Vis Sci 2011;88(7):881–889.
3. Ferris, F.L., Fine, S.L., Hyman, L., Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol 1984;102:1640–1642.
4. Olsen, T.W., Bottini, A.R., Mendoza, P., et al., The age-related macular degeneration complex: linking epidemiology and histopathology using the Minnesota Grading System (The Inaugural Frederick C. Blodi Lecture). Trans Am Ophthalmol Soc 2015;113:Blodi.
5. Gheorghe, A., Mahdi, L., Musat, O., Age-related macular degeneration. Rom J Ophthalmol 2015;59(2):74–77.
6. Spooner, K.L., Mhlanga, C.T., Hong, T.H., et al., The burden of neovascular age-related macular degeneration: a patient’s perspective. Clin Ophthal, 2018;12:2483–2491.
7. Hurley, S.F., Matthews, J.P., Guymer, R.H., Costeffectiveness of ranibizumab for neovascular age-related macular degeneration. Cost Eff Resour Alloc 2008; 12:6.
8. Sunness, J.S., The natural history of geographic atrophy, the advanced atrophic form of age-related macular degeneration. Mol Vis 1999;5:25.
9. Hageman, G.S., Anderson, D.H., Johnson, L.V., et al., A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to agerelated macular degeneration. Proc Natl Acad Sci U S A 2005;102(20):7227–7232.
10. Fett, A.L., Hermann, M.M., Muether, P.S., et al., Immunohistochemical localization of complement regulatory proteins in the human retina. Histol Histopathol 2012;27(3):357–364.
11. Boyer, D.S., Schmidt-Erfurth, U., van Lookeren Campagne, M., et al., The pathophysiology of geographic atrophy secondary to age-related macular degeneration and the complement pathway as a therapeutic target. Retina 2017;37(5):819–835.
12. van Lookeren Campagne, M., Strauss, E.C., Yaspan, B.L., Age-related macular degeneration: complement in action. Immunobiology 2016;221(6):733–739.
13. Fleckenstein, M., Mitchell, P., Bailey Freund, K., et al., The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology 2018;125(3):369–390.
14. Sadda, S.R., Guymer, R., Holz F.G., et al., Consensus definition for atrophy associated with age-related macular degeneration on OCT: classification of atrophy report 3.
Ophthalmology 2018;125(4):537–548.
15. Romond, K., Alam, M., Kravets, S., et al., Imaging and artificial intelligence for progression of age-related macular degeneration. Exp Biol Med (Maywood) 2021;246(20):2159–2169.
16. Sunness, J.S., Margalit, E., Srikumaran, D., et al., The long-term natural history of geographic atrophy from agerelated macular degeneration: enlargement of atrophy and implications for interventional clinical trials. Ophthalmology 2007;114(2):271–277.
17. Holz, F.G., Bindewald-Wittich, A., Fleckenstein, M., et al., Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol 2007;143(3):463–472.
18. Wang, J., Ying, G-S., Growth rate of geographic atrophy secondary to age-related macular degeneration: a metaanalysis of natural history studies and implications for designing future trials. Ophthalmic Res 2021;64(2):205–215.
19. Steinle, N.C., Pearce, I., Monés, J., et al., Impact of baseline characteristics on geographic atrophy progression in the FILLY trial evaluating the complement C3 inhibitor pegcetacoplan. Am J Ophthalmol 2021;227:116–124.
20. Lindblad, A.S., Lloyd, P.C., Clemons, T.E., et al., Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch Ophthalmol 2009;127(9):1168–1174.
21. Rosenfeld, P.J., Brown, D.M., Heier, J.S., et al., Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431.
22. Heier, J., Lad, M., Holz, F., et al., Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): two multicentre, randomised, double-masked, sham-controlled, phase 3 trials. The Lancet 2023;402(10411):1434–1448.
23. Apellis. Syfovre (pegcetacoplan injection) continued to demonstrate increasing treatment effects over three years in patients with geographic atrophy (GA). Press release, 4 Nov 2023. Available at: investors.apellis.com/newsreleases/news-release-details/syfovrer-pegcetacoplaninjection-continued-demonstrate-0#:~:text=SYFOVRE%20 continued%20to%20demonstrate%20increasing,to%20 the%20projected%20sham%20arm [Accessed 3 Feb 2024].
24. Apellis. Apellis provides updates on injection kits and rare safety events with GA drug Syfovre. Press release, 23 Aug 2023. Available at: investors.apellis.com/news-releases/news-release-details/apellis-providesupdates-injection-kits-and-rare-safety-events [Accessed 30 Jan 2024].