mieducation
Dry Eye Disease: Management Strategies for Every Optometrist
The diagnosis of dry eye disease can be confusing for patients, many of whom do not experience the classic ‘desiccated’ feeling on their eyeball surface, but rather complain of excessive wateriness of their eyes.
Alex Petty considers a practical approach to designing a dry eye disease clinical workup to ensure you’re giving your patients the best chance of improving their quality of life.
WRITER Alex Petty
LEARNING OBJECTIVES
On completion of this CPD activity, participants should be able to:
1. Design a clinical assessment plan to diagnose dry eye disease and other ocular surface diseases,
2. Understand the key clinical signs of dry eye disease and identify some of the ocular surface diseases that may coexist with, or mimic, the symptoms of dry eye disease, and
3. Understand how to select an appropriate lubricating eye drop for the different sub-types of dry eye disease.
Dry eye disease (DED) is a condition regularly encountered by eye care professionals around the world, which should come as no surprise considering that prevalence rates are reported to be as high as 50%. 1 Because of the chronic nature of the disease, and the range of symptoms associated with it, a DED diagnosis can be frustrating for many patients. This highlights the importance of appreciating the multi-factorial nature of ocular surface disease, and designing your DED clinical work-up in a way that will uncover the varying aetiologies and diagnoses behind patients’ symptoms.
This, in turn, will facilitate more appropriate treatment recommendations and ultimately give a better chance of improving quality of life in those individuals suffering from what can be an incessant burden.
We are fortunate to live in a time of rapid expansion of knowledge on ocular surface disease and dry eye topics. I will not attempt to summarise this colossal area in this article, but rather outline some of the key diagnostic and management considerations in ocular surface disease. Additionally, I will share some choice morsels of relevant information gleaned from the literature.
For those looking for a succinct summary of dry eye disease to complement this largely practical article, Dr Beata Sander’s ‘Dry Eye Disease: When Eye Drops No Longer Soothe’ in mivision April 2022 is an excellent read. 2
Of course, the comprehensive Tear Film and Ocular Surface DEWS II reports from 2017, 3 and the group’s recent Lifestyle report, 4 offer a wide-ranging and comprehensive deep-dive into this evolving field.
PATIENT HISTORY
When first assessing a patient with possible ocular surface disease, a good case history, although not glamorous, is crucial to elucidate possible risk factors contributing to their condition.
On occasion, it can give enough clues to tentatively diagnose some conditions without even inspecting the eyes. This has led some to suggest that it is appropriate to diagnose dry eye from symptoms alone, 5 however this is not the case for every patient; the same symptoms can be experienced with a range of ocular surface conditions and tear film disorders.
Risk factors can include a range of external environmental aspects such as lifestyle and workplace demands, the locations they spend time in, and the extent of their digital device use. Internal factors – such as medications, supplements and nutrition, and whether there’s been any previous ocular surgery,contact lens wear or trauma in the past – also play a role.
Meibum assessment, including digital and paddle/forceps expression
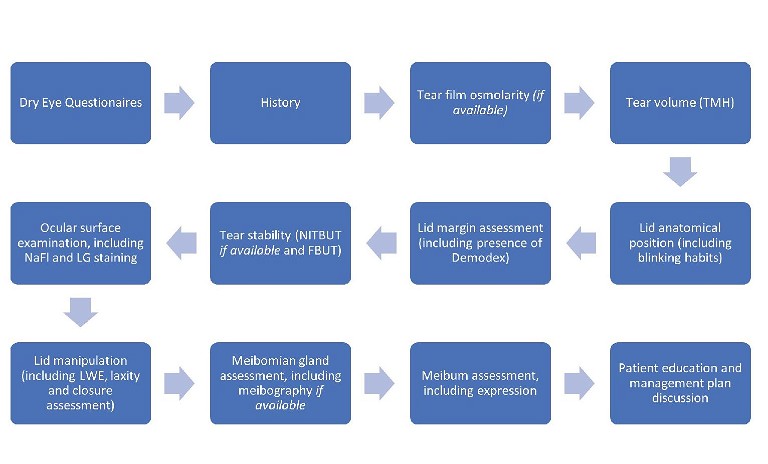
Figure 1. A flow chart of the recommended assessment process to evaluate dry eye and ocular surface disease.
Dry eye questionnaires are an effective and efficient way to rapidly collate information to better understand your patient’s history. These are typically completed by a patient prior to entering the consulting room.
A helpful strategy is to have front-of-house staff ask a few questions about dry eye disease symptoms when patients book or arrive for their appointments, and offer questionnaires to those who sound like they may have ocular surface problems. Some clinics will even offer the questionnaires to all patients, given how ubiquitous dry eye disease can seem when you really start looking for it. I have found that having a patient thinking about all aspects of their problem before even seeing you will also help them start to appreciate the potential complexity of their condition, which paves the way for them to invest time and effort into managing an often chronic condition, rather than just unrealistically expecting a quick fix.
A range of validated dry eye surveys exists, including the Ocular Surface Disease Index (OSDI), the longer 57 question Impact of Dry Eye on Everyday Life (IDEEL), and others such as the DEQ-5, SPEED, and SANDE. They will generally offer a score, which can be used to determine the risk of a patient having dry eye disease. For example, in the OSDI, patients can be categorised as having a normal ocular surface (0–12 points), mild (13–22 points), moderate (23–32 points), or severe (33–100 points) ocular surface disease. 6 Also, when questionnaires are repeated after treatments such as intense pulsed light therapy (IPL), this can objectively determine how well a patient has responded to management. Personally, I find the McMonnies questionnaire 7 particularly helpful for evaluating risk factors and rely more on the OSDI survey 8 to assess symptoms and response to treatment.
These surveys also provide a stepping stone into questions that delve deeper into a patient’s symptoms and risk factors. Some useful areas to explore include:
• When symptoms are most troubling (some conditions like floppy eyelid syndrome or nocturnal lagophthalmos may be worse on waking).
• What particular environments give the most symptoms (for example, patients are commonly most affected by wind, driving, and air-conditioning).
• How long symptoms have been present for (following cataract surgery is a common complaint in many older patients) and whether these vary during different times (ocular allergies may occur concurrently and contribute to more symptoms in spring/summer).
• What treatments have been tried in the past and what helped the most (many patients need gentle reminding about what they may have used previously, which helps to avoid offering a previously unsuccessful treatment or one that may be insufficient for their current disease state).
• What current treatments (including current oral medications and supplements) are being used, so as to better understand the logical next steps in their management journey.
STARTING THE OCULAR DISEASE WORK-UP
Once the patient history is complete, the clinical examination can begin. The steps in an ocular surface disease work-up should follow a logical pattern, starting from the least invasive to the most invasive, in order not to influence later observations and measurements.
Generally, I start by measuring tear film osmolarity. In our clinic we use the Tearlab System. This is best carried out before the natural tear integrity and volume is disturbed by lid manipulation, instillation of vital dyes, eye movements, or altered blinking during slit-lamp observation.
The DEWS II group recently updated the definition of dry eye disease to include tear film hyperosmolarity, 9 as this is the primary pathophysical event occurring in the condition. 10 Hyperosmolarity is a result of both mechanisms that contribute to dry eye disease, i.e., evaporative dry eye disease, which is excessive evaporation of the tear film due to meibomian gland dysfunction (MGD), and aqueous deficient dry eye (ADDE), which is reduced tear lacrimal gland production.
Values given by osmolarity instruments can provide clues about the patient’s ocular surface disease state. Generally, tear film osmolarity ≤308 mOsmol/L is considered normal, an osmolarity of 316 mOsmol/L is regarded as the threshold between mild and moderate-severe dry eye, and a difference of >8 mOsmol/L between eyes is believed to be suggestive of DED. 11 As with any measurements in medicine, results should be interpreted in context as studies demonstrate that some ‘normal’ patients will show elevated tear osmolarity. 12,13 In addition, conditions like allergic conjunctivitis, where we might expect normal tear film osmolarity, may actually demonstrate hyperosmolarity in some cases. 14
My next step is slit lamp assessment. First, I inspect the volume of the tear film by measuring the tear meniscus height (TMH). The tear meniscus is believed to contain 75–90% of the total volume of tears on the eye surface. 15 Low volumes can be suggestive of aqueous deficiency. The vertical height of the visible tear meniscus can be measured with your slit lamp beam or with automated imaging systems such as the Medmont Meridia (Figure 2) or Oculus Keratograph 5M (Figure 3).
A range of normal TMHs have been reported in the literature, ranging from between 0.2 and 0.5 mm depending on which study you read, suggesting that a value of <0.2 mm could be indicative of lacrimal deficiency. 16 If TMH is low, it can be helpful to confirm a low tear volume later in the work-up, for example with a phenol red thread test (PRTT).
If you suspect aqueous deficiency, especially in the context of a patient with a dry mouth or throat (handily prompted by the McMonnies questionnaire), it is a good idea to arrange a referral to their general practitioner or rheumatologist to run the recommended blood tests for Sjögren’s syndrome, the most common cause of aqueous deficiency.
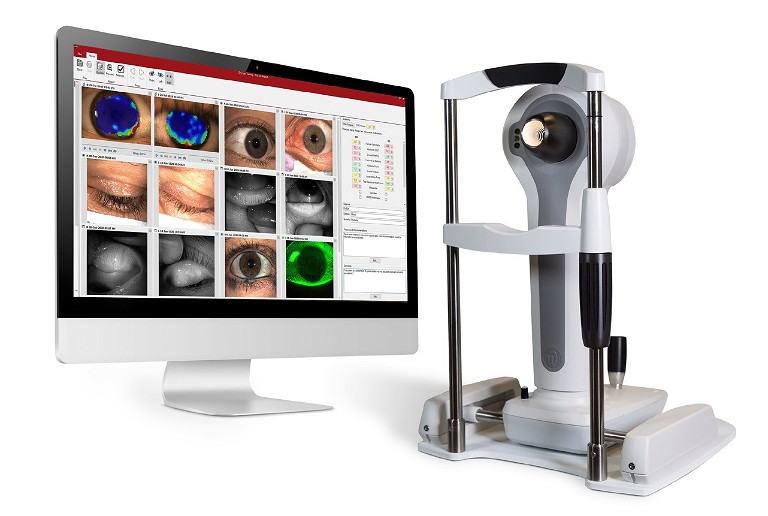
Figure 2. Medmont Meridia Advanced topographer.

Figure 3. Oculus Keratograph 5M advanced placido-based corneal topographer.
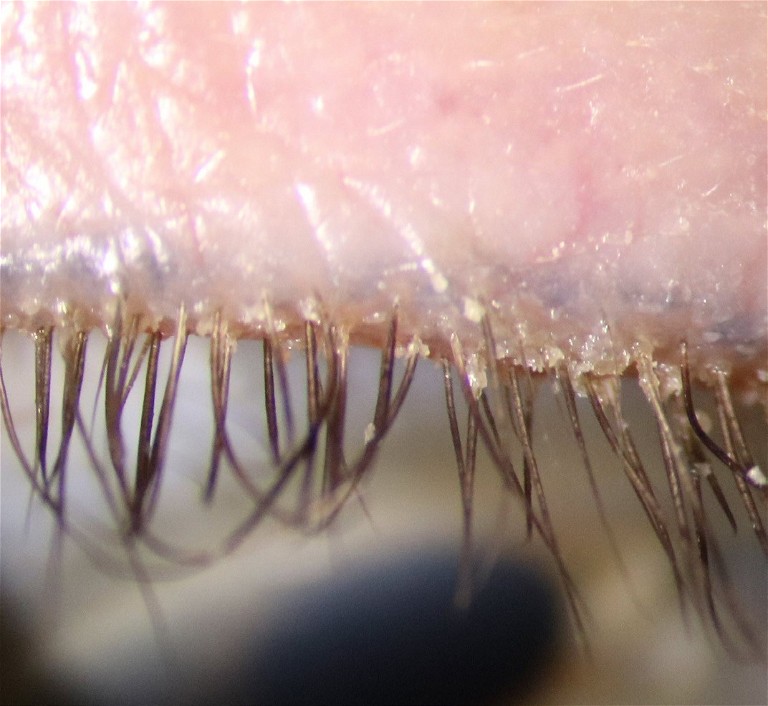
Figure 4. Cylindrical collarettes at the base of the lashes due to demodex anterior blepharitis. The presence of demodex was confirmed by inspecting an epilated lash on a slide with a video light microscope (a highly effective tool to encourage compliance with lid-care treatments when the patient can see the mites wriggling in real-time).
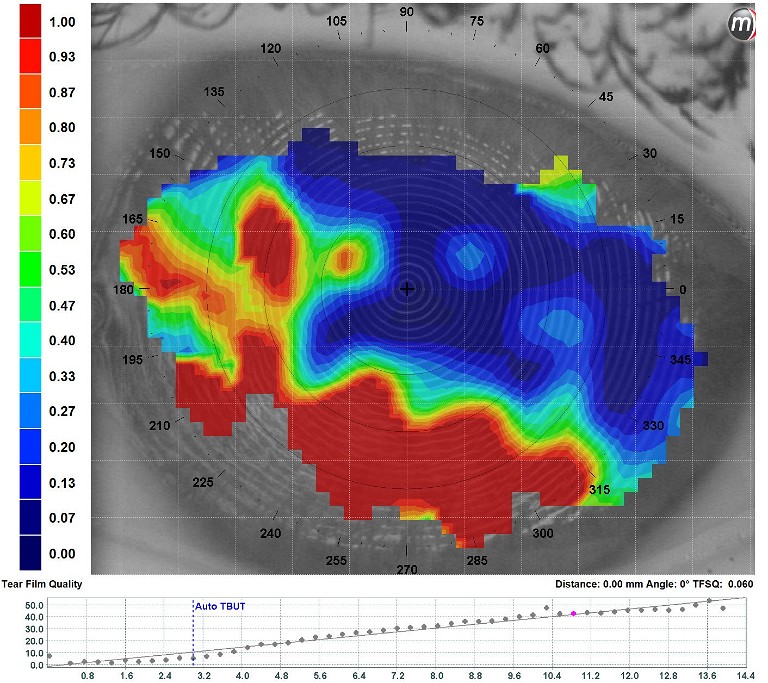
Figure 5. Automated non-invasive tear stability assessment with the Medmont E300, showing a rapid inferior destabilisation with NITBUT of three seconds and mire disruption (area with a dropout of the colour scale).
This will not necessarily change the ocular management, but can be helpful for other medical colleagues to diagnose and address any autoimmune conditions that become apparent. Be mindful that blood tests for the typical markers of Sjögren’s syndrome (SSA, SSB, ANA, and RF) only have sensitivity of around 75% and are not that reliable in the early stages of the disease process. 17
EYELID EXAMINATION
Next I assess the eyelids carefully. I inspect the lid position looking for signs of incorrect lid to globe interaction (ectropion or entropion), poor punctum apposition or narrow/closed /absent punctal openings, lid margin irregularity, or aberrant eyelash direction (trichiasis). If the lid position is less than ideal, then tears may drain poorly, leading to epiphora (watery eyes), transient vision changes, and retention of inflammatory mediators within the eye. It is worth explaining to patients (ideally with the use of clinical photos to demonstrate the issue) that even with improvements in other aspects of their ocular surface disease, poor lid anatomy may hold the patient back from alleviating all of their symptoms (in particular their watery eyes), without regular use of eye drops with tear-film stabilising properties, or surgical intervention.
Blepharitis
The appearance of the eyelid margin will indicate the presence of anterior blepharitis and posterior blepharitis (Figure 4).
Anterior blepharitis will be evident by the build-up of debris at the base of the eyelashes. Of particular relevance is the cylindrical collarettes formed by demodex mite overcolonisation. Recent reports suggest that 60–70% of patients with dry eye disease also present with demodex anterior blepharitis. 18,19 These patients will require concurrent treatment with lid cleaning products containing ingredients such as tea tree oil (TTO) derivatives that the resilient demodex mites are sensitive to, or even in-office lid cleaning with the likes of the BlephEx microdermal abrasion therapy.
Fortunately, there are a number of TTO lid care treatments available on the market now, making this condition a simpler one for patients to manage than in the past. Typically, this condition will return if treatment is stopped so it is recommended to keep patients on a maintenance level of lid cleaning and continue with regular monitoring in the clinic. A recent in vitro study suggests that the ingredients in TTO can harm meibomian glands, 20 however more research in a clinical setting is required. Until we know more, consider decreasing the frequency of application over time if the symptoms and signs of demodex blepharitis are controlled. Alternatively, consider other treatment options like intense pulsed light (IPL) or Manuka honey application that have potential to control demodex species .21
The inflammatory signs of posterior blepharitis and its most common form, meibomian gland dysfunction (MGD), will be apparent on the eyelid margin with erythema and the increased appearance of abnormal capillary vessels (telangiectasia, particularly apparent in patients with ocular rosacea), meibomian gland orifice capping (yellow mounds), lid margin notching (due to localised meibomian gland drop-out), and foaming or frothing (indicating meibomian oil saponification). It is not unusual to have asymmetrical levels of blepharitis in each eye, and it is typically worse in the eye that gives the patient the most grief symptomatically. Persistent asymmetric or unilateral disease that is not responsive to treatment should raise suspicion for carcinoma or immune-related lid disease such as discoid lupus erythematosus. 22
TEAR FILM BREAK-UP TIME
Before interacting with the lids further, I believe it is best to assess tear-film stability next by measuring tear-break-up time (TBUT); the time that elapses between a blink and the first signs of tear-film break up. Ideally, in a clinical setting this is done in a non-invasive manner (NITBUT) using placido-based topographers such as the aforementioned Meridia, K5 or older Medmont E300 (Figure 5).
These devices can monitor the disruption of the reflections of light from the tear film anterior surface as it destabilises, giving a repeatable and objective test. I always assess fluorescein break-up time (FBUT) as well, however I tend to give less weight to this measurement in my clinical decision making because the instillation of sodium fluorescein (NaFl) itself reduces the stability of the tearfilm. Objectivity is difficult due to variability in instillation and measurement techniques, and FBUT shows lower sensitivity and specificity for dry eye disease than NITBUT. 23
Using a saline-moistened fluorescein strip and shaking excess fluid from the strip before gently and briefly touching the tip to the conjunctiva, will provide less disturbance to the tear film. It will also avoid excess fluorescence, which can be inevitable when flooding the eye using a minim or vial of fluorescein.
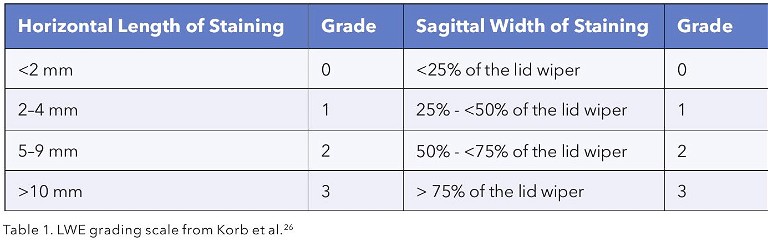
Anecdotally, the pattern of where fluorescein starts to break up first can give clues to the mechanism of epithelial disruption, for example inferiorly in those with incomplete blink or nocturnal exposure, even when frank epithelial NaFl staining has settled since the time of insult.
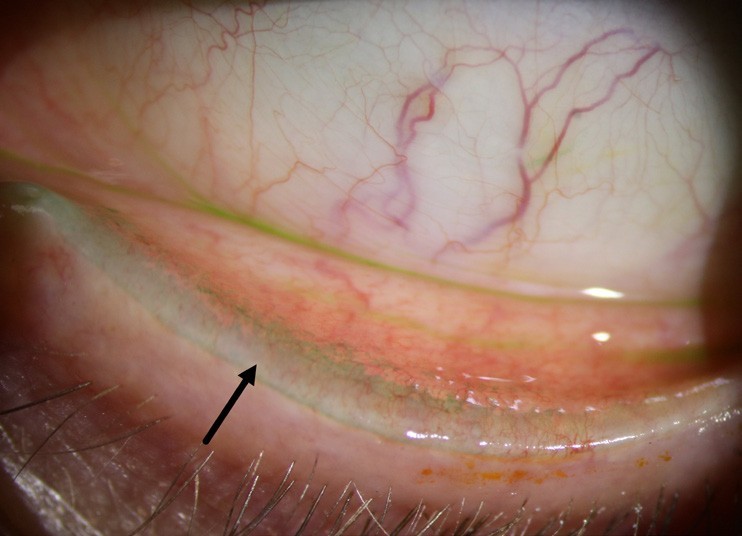
Figure 6. The inferior lid of a patient with grade three lid-wiper epitheliopathy, as shown by the extent of lissamine green staining.
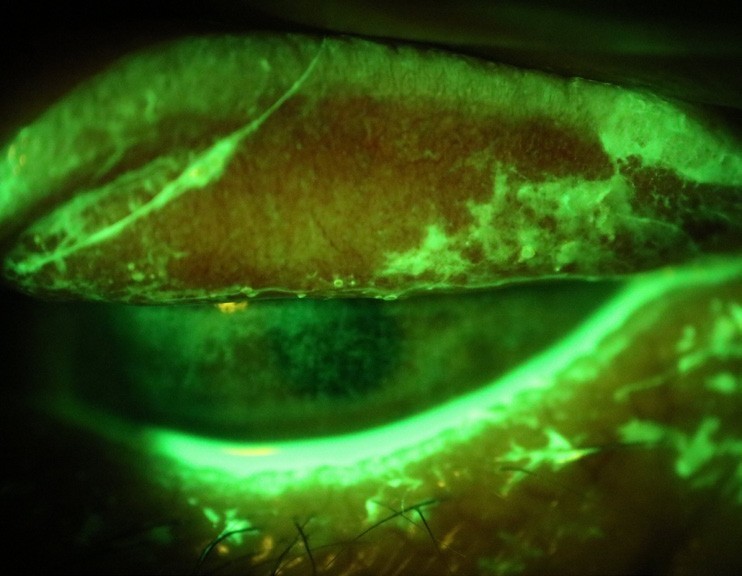
Figure 7. An everted superior lid with numerous exposed concretions showing up with fluorescein, viewed with a wratten filter. Note the corneal staining from abrasion and the protective mucous response.
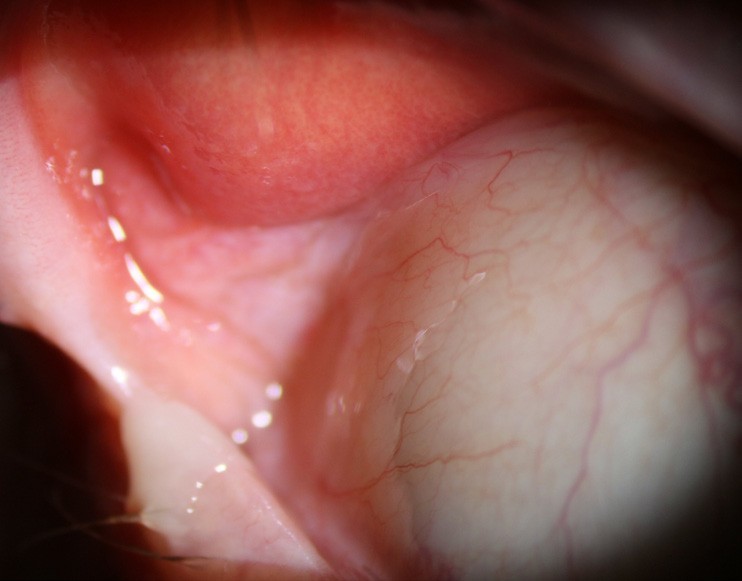
Figure 8. This photo shows the extent that a patient’s superior lid can be easily lifted up and off the globe in a case of floppy eyelid syndrome. Note the papillary inflammation on the palpebral conjunctiva.
EPITHELIOPATHY
Instillation of NaFL is important to assess corneal and conjunctival epitheliopathy (always with a Wratten filter for best sensitivity), and a crucial component when determining the severity and response to treatment of ocular surface disorder.
Lissamine green (LG) is another vital dye that is particularly useful for assessing conjunctival epitheliopathy and can also be helpful when identifying suspicious areas of conjunctiva with possible neoplastic features (Figure 6). Take care to leave one to five minutes after instillation of LG to allow maximal visualisation of conjunctival staining.24
It is at this stage that we need to manipulate the lids. Gentle eversion will show evidence of lid wiper epitheliopathy (LWE), a crucial diagnostic sign on both inferior and superior lid margins that indicates friction on the thin area of the palpebral conjunctiva that ‘wipes’ across the ocular surface. The normal lid wiper is rich in goblet cells and appears to be the most sensitive conjunctival tissue of the ocular surface.25 It is unsurprising that Korb et al. showed that 88% of symptomatic patients with DED had LWE but only 16% of asymptomatic patients presented with this sign.26
LWE tends to be less transient than corneal or bulbar conjunctival staining and is perhaps more of a reliable biomarker for the long-term state of a patient’s ocular surface disease: much like using HbA1c to evaluate a diabetic’s control over time is preferred to relying on a single blood glucose test.
As a patient’s ocular surface disease improves, so too should their LWE. Grading scales exist to help with monitoring LWE over time (Table 1), however they are no substitute for serial anterior segment photography (and patients love seeing evidence that their hard work is paying off clinically as well as symptomatically).
CHECKING FOR OTHER LESIONS AND FLOPPY EYE SYNDROME
Other lesions on the palpebral conjunctiva should be evident during your exam, such as exposed concretions, which are more common with MGD.27 Concretions are pieces of calcified cellular debris that have broken through the conjunctival surface, and occur more commonly than you would expect, in up to 7% of patients.28 Especially problematic under the superior lid, exposed concretions can cause considerable abrasive irritation to the eye surface if undetected.
Fortunately, NaFl will light up the particular pieces that have eroded, and generally these are easily removed with topical anaesthetic, a foreign body spud / tool / needle, and a steady hand.
When manipulating the lids to inspect the palpebral conjunctiva, make sure to check if there is significant laxity of the lids. If so, you should be able to pull them off the globe and see the fornices easily without eversion. This will often be associated with a fine papillar y conjunctivitis, which can be quite asymmetrical. This is a sign of the under-diagnosed condition, floppy eyelid syndrome (FES) (Figure 8). More common in obese males, FES occurs in around 3% of the population,29 and is due to a decrease in tarsal elastin. It can also be associated with other ocular conditions such as MGD and glaucoma. The main issue for FES sufferers is that the loose lids are easily pulled off the eye from the pressure of a pillow or bedding during sleep, leading to ocular surface exposure and inflammation.
History taking is crucial here. On questioning , patients will often realise they sleep on the side that they suffer more redness or morning irritation on. Symptoms will improve during the day as their lids resume more normal positioning; this is the opposite of most ocular disease sufferers. FES has a host of systemic associations, in particular obstructive sleep apnoea (OSA),30 a potentially serious health condition with cardiovascular consequences, where the throat muscles intermittently relax and block the airways during sleep. Patients with OSA will often be snorers, stop breathing for periods during sleep, or sleep poorly; waking feeling unrested or fatigued. Some patients with FES will already know about their OSA, and be using a continuous positive airways pressure (CPAP) machine during sleep. For others, it is recommended to refer to their GP to investigate further, sometimes with referral for sleep assessment.
There is a chicken and egg debate about the relationship between FES and OSA. In our clinic we have observed significant improvements in patients’ FES signs after diagnosis and treatment of their sleep apnoea, and this is also noted in the literature.31 Many, however, will require management of any coexisting dry eye disease, topical anti-inflammatory treatment, lubrication with thick gels or ointments before sleep, appropriate non-preserved topical artificial tears on waking, and in recalcitrant cases, lid taping or silicone eye shields to protect and isolate the ocular surface during the night.
BLINKING AND NOCTURNAL EXPOSURE
Another under-diagnosed problem related to correct eyelid behaviour concerns incomplete blinking, with resulting tear film evaporation and inferior exposure keratopathy. This can be particularly problematic when lagophthalmos occurs at night, when there is scant stimulus for reflex blinking. Again, history taking is needed to confirm a diagnosis (let’s be honest… very few eye care professionals will feel comfortable making house calls to inspect their patient’s nocturnal lid habits!). Patients will often report waking with irritated, red eyes, or are troubled by the feeling their lids are stuck tight on their eyeball on waking. Make sure to enquire with patients if they have had any previous lid or adnexa surgery, particularly blepharoplasty, as some will not associate their younger appearance with a tighter lid that no longer makes the same full excursions on blinking.
Blinking plays a significant role in maintaining homeostasis of the ocular surface, as blinking promotes lipid secretion from meibomian glands and the spreading of the stabilising oil across the ocular surface.32 Rather than asking them to ‘blink normally’, you can furtively observe a patient’s natural open-eye blink habit with the slit lamp (frequency and extent of upper lid excursion) by keeping them distracted with questions or observations about their eyes. Many patients are not aware of the deleterious effect on their blinking by their visual environment; one study for example showed computer use decreased blink rate to an average of four times per minute, a mere 20% of the rate of the same subjects during relaxed normal conversation.33
When assessing for risk of nocturnal exposure, there are a few helpful methods to employ. The Korb-Blackie lid light test involves lightly placing a transilluminator or bright penlight against the closed outer upper eyelid and observing the apparently closed lids for the presence of any light emanating from between them.34 Alternatively, when the patient has their eyes closed, ask them to look up while attempting to keep their eyes closed. This simulates the upwards eye movement during sleep caused by Bell’s phenomenon. Patients at risk of nocturnal exposure will show an area of exposed inferior conjunctiva.
Management of nocturnal exposure is similar to the protective goals of FES treatment, however without the need for as much of the anti-inflammatory elements. Incomplete blinking is a more multi-faceted condition to deal with, due to blink habits being related to a range of factors (atmospheric, visual tasks, personality type, alertness, corneal sensitivity) and the difficulty with significantly altering a subconscious action.
As Charles McMonnies observes in his excellent review on the topic of incomplete blinking,32 to train improved blink habits it is necessary to create an awareness of the significance of the problems associated with incomplete blinking and establish an appreciation of the advantages that are derived from normal complete blink habits. His Blink Instruction Guide recommends patients practise their blinks little and often; ideally every half an hour. In each session make 24 blinks consisting of three repetitions of eight blink cycles. On each blink within a cycle, one keyword is emphasised; full, complete, relaxed, light, quick , rapid, confident, natural.35 Patients that are diligent with their exercises should be able to decrease the proportion of inefficient blinks outside their training periods, with improved ocular surface function.
ASSESSING MEIBOMIAN GLAND
The final important step when evaluating the lids is to establish how dysfunctional the meibomian glands are. It is vital to visualise the glands themselves to look for changes to gland morphology or if frank gland atrophy has occurred. This can be done with shining a bright transilluminator back through the everted lid to observe the shadow of the meibomian gland outline, or ideally employing a device with infrared meibography to better visualise the glands in both inferior and superior lids, and more accurately document their appearance. Meibography abnormalities have been shown to be more common in those diagnosed with evaporative dry eye than aqueous deficient dry eye,36 and shortening of glands is more frequently detected in contact lens wearers that are symptomatic for DED.37
Do not be surprised when patients with significant meibomian gland atrophy have long histories of chronic dry eye symptoms, or have not had the expected improvements with normal MGD treatments like hot lid compresses and digital massage. These patients can be the hardest group to attain profound improvements with, as they have so little potential for normal meibomian gland function ( Figure 9).
I typically leave the meibomian gland expressibility assessment to last. Meibum is the stabilising lipid component released from meibomian glands, and its quantity, quality and expressibility directly relate to healthy meibomian gland function.38 In the normal eyelid, meibum is clear like olive oil and is readily expressed with gentle pressure with the tip of the finger against the lid margin.
Patients with MGD, however, will show meibum that is more cloudy, opaque, and even ‘toothpaste-like’ in its viscosity (obstructive MGD). Some patients, especially those with abnormal meibography, may show low or absent secretions (hyposecretory MGD). Despite it being somewhat unpleasant for the patient, it is important to use a lid-compression tool, such as a Mastrota paddle (a flat metal paddle which sits behind the lid as you press) or two-pronged expression forceps (which squeeze the lid from both sides and importantly can be operated with one hand), to properly evaluate the meibum features. This is important to help accurately diagnose the MGD subtype, which influences treatment recommendations. For example: some meibum can be clear with gentle expression, but thicker and cloudier deeper in the gland. Additionally, patients with an absence of meibum with gentle expression (suggesting loss of gland function), may actually have good meibum volume with firm expression pressure, and therefore better potential for functional improvement of the glands.
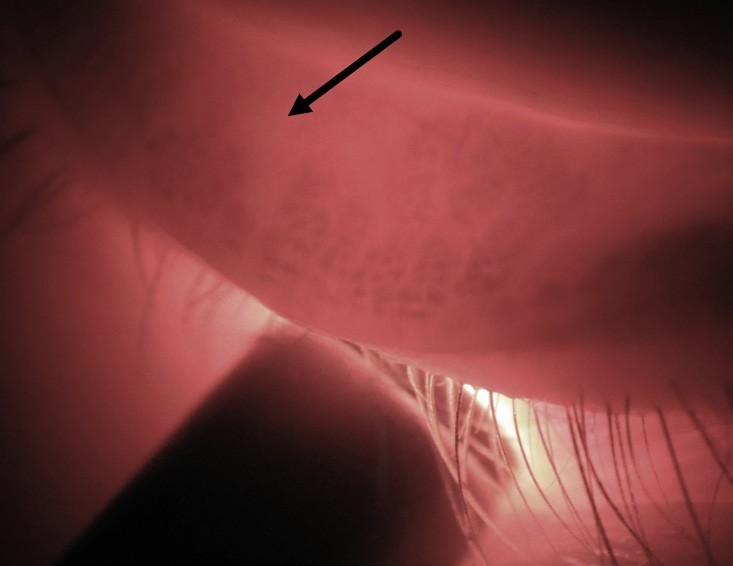
Figure 9. Transillumination of an inferior lid shows a moderate level of meibomian gland shortening due to atrophy, seen as the absence of dark acini structures. (image colour corrected).
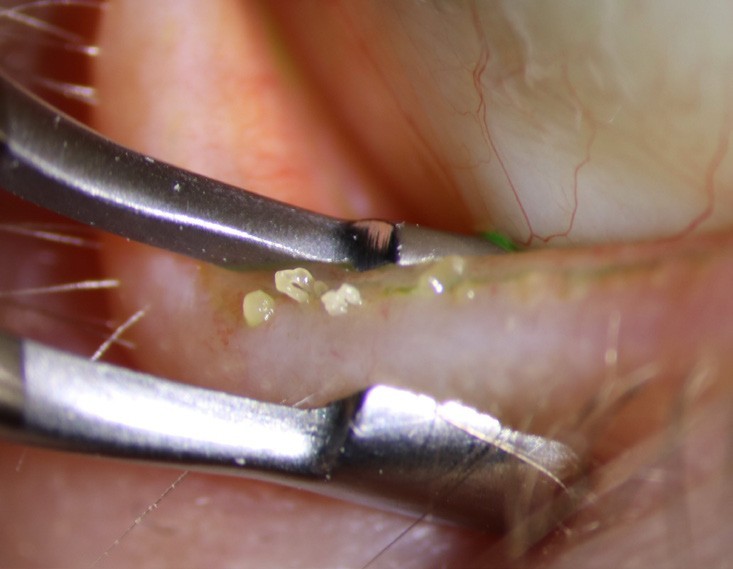
Figure 10. This image is typical of patients with severe obstruction MGD; meibum is as thick as toothpaste when firmly expressed with forceps.
Consider using a drop of topical anaesthetic to ease patient awareness of your tools against the palpebral conjunctiva (and accidental glancing touches on the globe) and do your best to express the superior glands too. Anecdotally, the latter seem to perform better than the inferior glands, despite being harder and more unpleasant to apply pressure to. This is possibly due to the stronger orbicularis oculi muscle pressure working in this area (Figure 10).
DIAGNOSIS
From this work-up you should be able to confidently diagnose the patient’s ocular disease state; whether they have dry eye disease, and what subtype; whether any other ocular surface conditions like lagophthalmos, floppy eye lid syndrome, ocular rosacea or anterior blepharitis are present; the severity of the condition, and the resultant inflammation that inevitably results. Let’s explore a quick case to highlight a real-world example of this workup, and how this influences treatment options.
CASE REPORT
Mr Eton,* a 76-year-old retiree from a town several hours drive away, was referred to our clinic by his general ophthalmologist to get our thoughts on the ocular surface disease problems that had been troubling him for years.
His key complaints were that his eyes would get a crusty build up, and be stuck together in the mornings. During the day they were constantly irritable and watery. Air-conditioning and dry areas would particularly aggravate him. He was otherwise reasonably healthy although suffered from migraines (for which he took Nadalol), used omeprazole for acid reflux, and took multivitamins and 2,000 mg of omega-3 containing fish oil supplements daily. On further questioning , he admitted that he had always slept poorly, often waking grouchy and unrested.
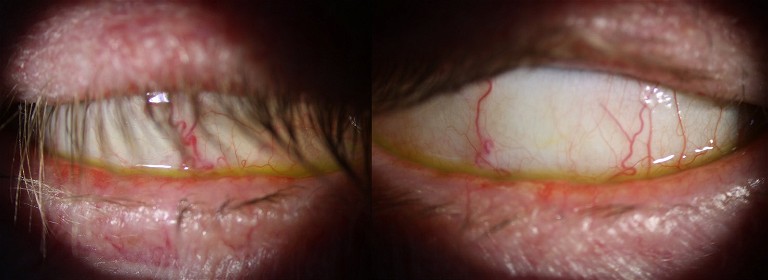
Figure 11. An image of Mr Eton, showing his exposed ocular surface when asked to close his eyes and look up, suggesting he is prone to nocturnal ocular surface exposure.
His current treatments to manage his eye problems included daily baby-shampoo lid margin cleaning , and 1 mg/mL sodium hyaluronate ( Hylo-fresh) non-preserved lubricant eye drops, varying his use from several a day to many.
His McMonnies questionnaire gave a score of 22 (suggestive of dry eye), and his OSDI survey score was 75/100 (suggesting severe dry eye). Aided vision was reasonable in each eye at 6/7.5. Tear film osmolarity with Tearlab showed normal levels in each eye (R 283 mOsmol/L, L 281 mOsmol/L), implying factors other than DED may be at play. His tear-meniscus height was normal at 0.4 mm. His eyelashes showed evidence of mild cylindrical collarettes and each lid margin had moderate levels of telangiectasia. His lids appeared to be positioned normally against the globe, with no ectropion or punctum abnormalities. Non-invasive tear-break up time (Medmont E300) was normal at >15 seconds. His corneas showed no significant staining with NaFl or LG, and there was no significant lid-wiper epitheliopathy (Grade 0). With manipulation, however, both lids showed significant laxity and it was possible to lift the lids right off the globe, exposing the inflamed papillar y appearance of the palpebral conjunctiva. Furthermore, when I asked the patient to close his eyes and look up, his lids were still open by ~3–4 mm, exposing the inferior bulbar conjunctiva ( Figure 11). Despite this, his blinking habits seemed reasonably normal, with full excursions. With transillumination his meibomian glands showed no obvious dropout, and his meibum was abundant, however was significantly cloudy with digital expression. With more forceful forceps expression, he showed thicker ‘toothpastelike’ expressions. The remainder of the ocular examination was unremarkable.
Several different issues contributed to Mr Eton’s ocular surface disease:
• Obstructive meibomian gland dysfunction (severe due to the poor meibum quality and degree of lid margin inflammation) with secondar y evaporative dry eye disease (surprisingly at only a mild level as indicated by normal tear osmolarity, NITBUT, and corneal appearance),
• Demodex anterior blepharitis,
• Probable nocturnal ocular surface exposure, and
• Floppy eyelid syndrome, with likelihood of further ocular surface exposure.
Discussion
The range of disease mechanisms explains the chronicity of his condition and the lack of improvement with his existing treatment plan. Management in these cases first involves patient education, ideally with the use of clinic images, diagrams or eye models. The patient was surprised to learn of the range of issues at play, but was relieved to understand the reasons for his symptoms.
It is important to treat each component of the patient’s ocular surface disease to have the best chance of improving symptoms and breaking the cycle of inflammation. To tackle his meibomian gland dysfunction, we prescribed an anti-inflammatory dose of oral azithromycin tablets (500 mg on the first day, then 250 mg once a day for four more days) as per the 2015 Kashkouli et al. study39 that showed azithromycin had a better clinical response when treating MGD compared to oral doxycycline, with fewer side-effects and a shorter course. We recommended he continue his omega-3 supplements due to the beneficial effect they have on meibomian gland function and ocular inflammation, and we prescribed an oil-containing lubricating eye drop ( Novatears, containing perfluorohexyloctane 100% v/v;) to be used four times a day.
The choice of daily lubricant eye drops for symptomatic support is a crucial component of management, despite the fact that these drops are not fundamentally improving the root cause of their condition. If a patient has aqueous deficient dry eye disease, then a watery drop is best, however if evaporative dry eye disease is present, the patient is likely to do best when the drop has an oily component that mimics the natural meibum and improves tear stability.
The GOBI study40 evaluated the efficacy and safety of perfluorohexyloctane ophthalmic solution in patients with dry eye due to MGD. From as early as two weeks, Novatears demonstrated statistically significant and clinically meaningful improvements in the signs and symptoms of DED, when compared with placebo. Other studies have also shown similar improvements in tear stability, corneal staining , and OSDI scores with perfluorohexyloctane drops.41 We have used Novatears in our clinic for over five years, and found that patients have generally preferred the control this drop provides when compared with other drops of an aqueous nature. Also, no preservative is required because bacteria cannot live in the semifluorinated alkane medium, which makes it ideal for regular use in chronic dry eye.
Novatears is also particularly useful when there is limited improvement expected in the underlying disease mechanism, and regular addition of drops to supplement the stability of their tear-film are required, for example in those with atrophic MGD, epiphora caused by reflex tearing in the context of ectropion, or incomplete blinking. Make sure to warn patients of the smaller volume drop than they are used to (approximately one third of a standard drop) and the ‘warm’ or ‘soft’ feel of the low-surface tension drop when it hits the eye. Initially some patients will be unsure if they have instilled enough, even though a single drop is ample.
Improving the meibomian gland function should help with the nocturnal exposure in these patients, as a more robust tear-film will resist the drying effects during the night, however extra nightly lubrication is often needed. We recommended a liberal amount of Vit A-POS ointment (138 µg/g retinol palmitate) be applied to the eye before sleep, and suggested that using a drop of Hylo-Fresh on waking would be appropriate to flush out the eyes of debris to start the day. Because of the lack of corneal or conjunctival staining , we decided not to prescribe any anti-inflammatory drops like fluorometholone acetate 0.1% or nonpreserved methyl-prednisolone 1% for Mr Eton’s FES at that time. We did recommend the patient replace his baby-shampoo with a tea-tree oil containing eyelid cleanser foam to be scrubbed into the eyelashes daily (Ocusoft Oust). Finally we copied the patient’s general practitioner into the letter to his referrer, and suggested, given the FES diagnosis and his histor y of sub-par sleep, that they investigate obstructive sleep apnoea.
The patient returned approximately one month later doing reasonably well. He had been diligent with his treatment regimen and was finding that his eyes were no longer crusty in the morning , and his daytime irritation and wateriness was less. Clinically, the collarettes on his lashes had reduced, and his meibum quality had improved, with the oils flowing easier despite still being cloudy, and with good volume when digitally expressed. His ocular surface remained clear of staining , however as expected, his lids were still very loose. The patient was pleased, but was keen for further improvement in his day-time comfort. To complement his ongoing treatments, we recommended a course of IPL therapy to help his meibomian gland function, which at the time of writing was underway.
CONCLUSION
Dry eye and ocular surface disease are a common problem; our patients deserve a comprehensive analysis of the often complex and varied mechanisms of their condition. Hopefully this article has shown that, even without access to all the newest diagnostic technologies available, using a logical and robust assessment process will make correct diagnosis of your patient’s condition(s) more straight-forward. This in turn, will facilitate appropriate treatment and management strategies, ultimately, leading to happier patients who will sing your praises to other potential dry eye sufferers needing your care.
*Patient name changed for anonymity.
The author received a monetary honorarium from AFT Pharmaceuticals for providing this article to mivision.
To earn your CPD hours from this article visit: mieducation.com.au/dry-eye-diseasemanagement-strategies-for-every-optometrist.
References
1. Stapleton, F., Alves, M., Bunya, V.Y., et al., TFOS DEWS II epidemiology report. Ocul Surf. 2017 Jul;15(3):334–365. DOI: 10.1016/j.jtos.2017.05.003.
2. Sander, B. Dry Eye Disease: When Eye Drops No Longer Soothe. mivision 2022. 177. Available at: mieducation.com/pages/dry-eye-disease-when-eye-drops-no-longer-soothe [accessed 31 Jan 2024].
3. Craig, J.P., Nelson, J.D., Azar, D.T., et al., TFOS DEWS II report executive summary. Ocul Surf. 2017 Oct;15(4):802- 812. DOI: 10.1016/j.jtos.2017.08.003.
4. Craig, J.P., Alves, M., Wolffsohn, J.S., et al., TFOS lifestyle report executive summary: a lifestyle epidemic - ocular surface disease. Ocul Surf. 2023 Oct;30:240–253. DOI: 10.1016/j.jtos.2023.08.009.
5. Schein, O.D., Munoz, B., Tielsch, J.M,et al., Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997;124:723–728.
6. Walt, J. Ocular Surface Disease Index (OSDI) Administration and Scoring Manual. Irvine, CA Allergan, Inc 2004.
7. McMonnies, C.W., Key questions in a dry eye history. J Am Optom Assoc. 1986 Jul;57(7):512–7.
8. Schiffman, R.M., Reliability and validity of the ocular surface disease index. Archives of Ophthalmology. 2000;118(5):615–621.
9. Craig, J.P., Nichols, K.K., Akpek, E.K., et al., TFOS DEWS II definition and classification report. The Ocular Surface. Jul 2017;15(3):276–283. DOI: 10.1016/j.jtos.2017.05.008.
10. Messmer, E.M., Pathophysiology of dry eye disease and novel therapeutic targets. Experimental Eye Research. Apr 2022;217:108944.
11. Greiner, J.V, et al., Association of tear osmolarity with signs and symptoms of dry eye disease in the dry eye assessment and management (DREAM) study. Invest Ophthalmol Vis Sci. 2023 Jan 3;64(1):5.
12. Messmer, E.M., Bulgen, M., Kampik, A., Hyperosmolarity of the tear film in dry eye syndrome. Dev Ophthalmol. 2010; 45:129–138.
13. Szalai, E., Berta, A., Szekanecz Z, Szucs G, Modis L.. Evaluation of tear osmolarity in non-Sjögren and Sjögren syndrome dry eye patients with the TearLab system. Cornea . 2012; 31: 867–871.
14. Nitoda, E., Lavaris, A., Laios, K., et al., Tear film osmolarity in subjects with acute allergic rhinoconjunctivitis. In Vivo. 2018 Mar–Apr;32(2):403–408.
15. Holly, F.J., Physical chemistry of the normal and disordered tear film. Trans Ophthalmol Soc U K. 1985;104:374–380.
16. Doughty, M.J., Laiquzzaman, M., Oblak, E., Button, N., The tear (lacrimal) meniscus height in human eyes: A useful clinical measure or an unusable variable sign? Cont Lens Anterior Eye. 2002 Jun;25(2):57–65.
17. Shen, L., Malyavantham, K., Suresh, L., et al; Early detection of Sjögren’s syndrome; Sensitivity and specificity of the Sjö diagnostic test. Invest. Ophthalmol. Vis. Sci. 2016;57(12):5681.
18. Cheng, A.M., Hwang, J., Dermer, H., et al., Prevalence of ocular demodicosis in an older population and its association with symptoms and signs of dry eye. Cornea 2021;40:995–1001.
19. Trattler, W, Karpecki, P., Rapoport, Y., et al., The prevalence of Demodex blepharitis in US eye care clinic patients as determined by collarettes, a pathognomonic sign. Clin Ophthalmol 2022;16:1153–1164.
20. Chen, D., Wang, J., Sullivan, D.A., et al., Effects of Terpinen-4-ol on meibomian gland epithelial cells in vitro. Cornea. 2020 Dec;39(12):1541–1546.
21. Frame, K., Cheung, I.M.Y., Wang, M.T.M., et al., Comparing the in vitro effects of MGO Manuka honey and tea tree oil on ocular Demodex viability. Cont Lens Anterior Eye. 2018 Dec;41(6):527–530.
22. Gloor, P., Kim, M., McNiff, J.M., Wolfley, D., Discoid lupus erythematosus presenting as asymmetric posterior blepharitis. Am J Ophthalmol. 1997 Nov;124(5):707–9.
23. Wolffsohn, J.S., et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017 Jul;15(3):539–574.
24. Lievens, C.W., Norgett, Y., Briggs, N., et al., Optimal methodology for lid wiper epitheliopathy identification. Cont Lens Anterior Eye. 2021 Jun;44(3):101332.
25. Varikooty, J., Srinivasan, S., Subbaraman, L., et al., Variations in observable lid wiper epitheliopathy (LWE) staining patterns in wearers of silicone hydrogel lenses. Cont Lens Anterior Eye. 2015 Dec;38(6):471–6.
26. Korb, D.R., Herman, J.P., Blackie, C.A., et al., Prevalence of lid wiper epitheliopathy in subjects with dry eye signs and symptoms. Cornea. 2010 Apr;29(4):377–83. DOI: 10.1097/ICO.0b013e3181ba0cb2.
27. Haicl, P., Janková, H., Jirsová, K., Syndrom suchého oka u nemocných se spojivkovými konkrementy [Dry eye syndrome in patients with conjunctival concretions]. Cesk Slov Oftalmol. 2006 Nov;62(6):415–22.
28. Kulshrestha, M., Thaller, V., Prevalence of conjunctival concretions. Eye. 1995;9:797–798. DOI:10.1038/EYE.1995.197.
29. Kadyan, A., Asghar, J., Dowson, L., Sandramouli, S., Ocular findings in sleep apnoea patients using continuous positive airway pressure. Eye (Lond). 2010 May;24(5):843- 50. DOI: 10.1038/eye.2009.212.
30. Cheong, A.J.Y., Ho, O.T.W., Wang, S.K.X., et al., Association between obstructive sleep apnea and floppy eyelid syndrome: A systematic review and metaanalysis. Surv Ophthalmol. 2023 Mar–Apr;68(2):257–264. DOI: 10.1016/j.survophthal.2022.11.006.
31. Chan, J.S., Lee, M.K., Tweedie, P.J., Observations on the association between obstructive sleep apnea and floppy eyelid syndrome: A systematic review and meta-analysis. Surv Ophthalmol. 2023 Oct 7:S0039–6257(23)00130-3. DOI: 10.1016/j.survophthal.2023.10.001.
32. McMonnies, C.W., Incomplete blinking: exposure keratopathy, lid wiper epitheliopathy, dry eye, refractive surgery, and dry contact lenses. Cont Lens Anterior Eye. 2007 Mar;30(1):37-51. DOI: 10.1016/j.clae.2006.12.002.
33. Patel, S., Henderson, R., Bradley, B., et al. Effect of visual display unit use on blink rate and tear stability. Optom Vis Sci 1991 Nov;68:888–92. DOI: 10.1097/00006324- 199111000-00010.
34. Blackie, C.A., Korb, D.R., A novel lid seal evaluation: the Korb-Blackie light test. Eye Contact Lens. 2015 Mar;41(2):98–100. DOI: 10.1097/ICL.0000000000000072.
35. McMonnies, C.W., Blinking: dry eyes and dry contact lenses. Sydney: Institute for Eye Research; 2006.
36. Arita, R., Itoh, K., Maeda, S., et al., Efficacy of diagnostic criteria for the differential diagnosis between obstructive meibomian gland dysfunction and aqueous deficiency dry eye. Jpn J Ophthalmol. 2010 Sept;54(5), 387–391 DOI: 10.1007/s10384-010-0858-1.
37. Arita, R., Itoh, K., Inoue, K., et al., Contact lens wear is associated with decrease of meibomian glands.Ophthalmology. 2009 Mar;116(3):379–84. DOI: 10.1016/j. ophtha.2008.10.012.
38. Wolffsohn, J.S., Arita, R., Chalmers, R., et al., TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017 Jul;15(3):539–574. DOI: 10.1016/j.jtos.2017.05.001.
39. Kashkouli, M.B., Fazel, A.J., Kiavash, V., et al., Oral azithromycin versus doxycycline in meibomian gland dysfunction: a randomised double-masked open-label clinical trial. Br J Ophthalmol. 2015 Feb;99(2):199–204. DOI: doi.org/10.1136/bjophthalmol-2014-305410.
40. Tauber, J., Berdy, G.J., Wirta, D.L., Krösser, S, Vittitow JL; GOBI Study Group. NOV03 for dry eye disease associated with meibomian gland dysfunction: Results of the randomized phase 3 GOBI study. Ophthalmology. 2023 May;130(5):516–524. DOI: 10.1016/j.ophtha.2022.12.021.
41. Steven, P. Augustin, A.J., Geerling, G., et al., Semifluorinated alkane eye drops for treatment of dry eye disease due to meibomian gland disease. J Ocul Pharmacol Ther. 2017 Nov;33(9):678–685. DOI: 10.1089/ jop.2017.0042.

Alex Petty BOptom (Hons) FIAOMC is a therapeutic optometrist, contact lens specialist, and orthokeratologist. He is the director and principal optometrist at Bay Eye Care, a medically-focussed specialty optometry clinic in Tauranga, New Zealand.
He also has interests in myopia control and ocular disease management, including dry eye and glaucoma.
He has lectured locally and internationally on contact lenses and ocular disease and has published a number of articles and case reports in clinical optometry journals and education forums.
In 2016, Mr Petty was one of the first New Zealanders to become a fellow of the International Academy of Orthokeratology and Myopia Control (IAOMC). He has previously held the role of Vice-President of the Orthokeratology Society of Oceania, and section fellowship chair for Oceania for the IAOMC.