mipatient
Looking to the Future of Dry Eye Management
WRITER Dr Margaret Lam
Emerging dry eye treatments have the potential to improve our patients’ quality of life. Dr Margaret Lam spoke to optometrist, educator and dry eye enthusiast Martin Robinson about where he feels dry eye management is today, and where it can be in the future.

Dr Margaret Lam

Martin Robertson
“ But no matter what technology we have... patients will always need the empathy and dedication of practitioners ”
Q. How do you think dry eye management has evolved? What have we become better at over the past few decades?
Dry eye management and treatment has evolved exponentially since the 1990s. While the pace of change was slow during the 1990s, and we saw just a little more progress in the 2000s, we went ahead with leaps and bounds in the 2010s, and so much so in the 2020s that it is hard to even imagine all the pipeline drugs and treatments now emerging.
When I started work , preserved eye drops, warm compresses, and lid scrubs with baby shampoo were about the extent of dry eye treatments. All three are viewed as somewhat suspect now; preservative exposure is to be limited, warm compresses are to be avoided in ocular rosacea and when significant gland obstructions and stasis are present, and we know baby shampoo is inflammatory.
We recognise the multifactorial nature of dry eye disease (DED), and we have identified the effects of preservatives, demodex and bacteria, environmental conditions, and behaviour, and perhaps most importantly, the impact of inflammation. As a consequence, we now have multiple ways to reduce inflammation; from a top-down approach using hypochlorous acid, intense pulsed laser ( IPL), and low level light therapy (LLLT), to a bottom-up approach, using steroids, ciclosporins, azithromycin, and doxycycline.
Equipment to help us identify signs is becoming more commonplace and more accurate.
Some devices are using machine learning and artificial intelligence (AI) to interpret dynamic functions of the tear film. AI algorithms are being used to assess tear break-up, to analyse blink patterns, and interpret meibography. AI tools are very recent developments in the field of dry eye, but there is amazing potential to enhance the speed and accuracy of diagnosis. The same can be said of acoustically-driven microfluidic extensional rheometry (ADMiER ), a revolutionary device developed by Professor Laura Downie and colleagues at The University of Melbourne, that can classify the subtype of dry eye your patient has.
But no matter what technology we have, or what tools we use, patients will always need the empathy and dedication of practitioners.
Q. What are the most common undiagnosed dry eye conditions you see that cause the greatest impact on a patient’s quality of life?
That is probably not the question to ask, as most referrals are accurate, with the majority of dry eye signs and symptoms, and an accurate subtype identified.
The problem, as I see it, is still the mismatch of treatments prescribed to treat the signs and symptoms. This definitely negatively impacts a patient’s quality of life. For example, I have seen hyperkeratinisation of the lid margin identified with warm compresses being the only treatment prescribed. This is analogous to your dentist diagnosing scale and tartar, but prescribing mouthwash to treat it. If we follow the analogy, your dentist knows home therapy is not adequate to treat tartar, so they prescribe and perform a scale and clean in office, then prescribe good toothbrushing and mouth washing for home maintenance, and send a reminder for review in six months as they know the tartar will be back .
If you see a clinical sign, then treat it the best way you can. In the case of hyperkeratinisation, prescribe a lid debridement in clinic, then prescribe home maintenance of hypochlorous acid sprays / tea-tree foam / warm compresses. And most importantly, schedule appropriate reviews. As eye care professionals, we are best placed to remove collarettes/biofilm in clinic; we will always clean the eyelids better in one session than our patients will in a few weeks.
If you find any abnormal sign, treat that sign. If the patient describes an abnormal symptom, provide treatment to reduce that symptom. This will have the most impact on your patient’s quality of life.
Q. From your extensive experience managing dry eye, what three tips can you share to help other eye care practitioners in the early detection and management of DED?
Tip 1. Check for incomplete blinking. This is by far the most common sign I find in my dry eye examinations, and yet I often hear fellow practitioners say they never see it. If you never see it, I suggest you look deeper. Watch the blink patterns with fluorescein and you will see the blinks often stop short and leave a tell-tale dark blink line at the lowest point reached. Watch the lower lid margin, and you will see it rarely gets touched by the upper lid.
Tip 2. Check for bacterial signs. Frothy bubbles, biofilm plaque on lid margins and under eyelashes. Bacteria perhaps play a larger role than demodex, and even demodex are bacterial vectors. With the widespread availability of hypochlorous acid sprays, this is something almost every optometrist can offer to help patients. These sprays make up a significant percentage of my prescribing. Lid debridement and lid/lash cleaning make up a significant part of the procedures I perform in clinic.
Tip 3. If your patient describes burning or stinging eyes, suspect the presence of hyperosmolarity. Advice for your patients should include increasing water intake, reducing salt in their diet, using a low osmolarity eye drop like one containing hyaluronic acid, and reducing alcohol intake, especially in women where it has a more significant impact.
Q. Can you share your three favourite tips for communication to help achieve patient compliance with dry eye management?
It’s hard to limit this to three, but here they are. They expand on my three previous tips.
Tip 1. Reinforce the importance of blinking properly. I do this in two ways. Firstly, I explain and demonstrate how when we blink, the eyelids do not simply close; rather they slide/zip close (this is something we should all remember from toric contact lens rotations).
In addition, the upper lid undergoes a degree of inward rotation, which is why lid debridements can assist in improving blink comfort.
With a normal full blink, the combination of both actions opens the meibomian acini and helps clean the lid margins. With an incomplete blink, the same forces are applied to the corneal and conjunctival surface, resulting in trauma, and increased inferior staining. This is a valid reason for blink exercises and provides great motivation in most patients.
Secondly, I advise patients to find time to practise blinking. I suggest opportunities, like every time a patient boils the kettle, or while they do yoga. When doesn’t matter, they just need to find an activity/time that works for them and do it!
Tip 2. I start the discussion about lid hygiene with questions: ‘How many times a day do you wash your hands? How many times a day do you brush your teeth? How many times a week do you wash your hair? Can you remember the last time you cleaned your eyes, and how?’
The answers show the disparity in eye hygiene vs everything else. I then explain how a once-a-day hypochlorous acid spray is the least we can do to keep our eyes’ bacterial flora under control, and it is not too much work.
Tip 3. Most patients use their eye drops in a reactionary way, in response to their eyes stinging or burning. To help with compliance, I stress preventative use: regular eye drops are like regular medication. Patients who experience chronic pain/period pain are fully aware of the benefits of regular medication. I explain that eye drops should be considered a way to prevent friction and reduce inflammation and as such, need to be used regularly. If I am prescribing three times a day, I advise my patients to use their drops at breakfast, lunch, and dinner. If I am prescribing drops five times a day, I recommend breakfast, morning tea, lunch, afternoon tea, and dinner. It may be every time they eat, or every time they have a cuppa. Again, they just need to find something they do regularly and use the drops at the same time.
Q. What aren’t we getting quite right yet to improve the management of dry eye for our patients?
I think we can all do better with our physical exam, especially our slit lamp examination. This helps with accurate diagnosis of offending signs and allows correct treatments to be prescribed.
There is a great mantra to remember: L.L.P.P.E.P.R. Look, Lift, Push, Pull, Educate, Prescribe, Review. Often, I have patients referred to me with a clinical sign not identified or treated prior. These are most often a sign that can be seen on slit lamp alone, and if identified and treated earlier, would have helped the patient and removed the need for referral to a dry eye practitioner.
Q. Where do you see dry eye disease diagnosis and treatment improving in the future?
As mentioned, there are constant improvements in equipment for diagnosis and treatment. The pipeline drugs are very promising, and the devices and equipment being developed are exciting as well.
Some of the emerging drugs to keep an eye on include:
• The use of topical insulin for dry eyes, diabetes, and neurotrophic keratitis. 1
• CBT-008 methyl beta cyclodextrin to dissolve cholesterol crystals at the orifices of meibomian glands (phase 2 trials). 2
• AZR-MD-001 selenium sulphide ophthalmic ointment. This is both a keratolytic and keratostatic agent that encourages lipogenesis and improves meibomian gland function. 3
• Aldeyra-reproxalap, a reactive aldehyde species (RASP) inhibitor in clinical trials for DED and allergic conjunctivitis. 4
• Xdemvy-lotilaner ophthalmic solution. This is approved by the United States Food and Drug Administration and available in the United States as a topical treatment for demodex. 5
Some of the new devices/technologies being promoted and developed worldwide include:
• Radio frequency devices, which are being used overseas to treat meibomian gland dysfunction.
• Acoustically driven microfluidic extensional rheometry (ADMiER), a single test to diagnose the subtype of dry eye.
• CSI dry eye software, a machine learning cloud-based software designed and created by dry eye practitioners.
Not all of these are available in Australia, but these examples showcase the advances occurring all around the world and evidence my view that dry eye is an ever-increasing area of research.
“ There is a great mantra to remember: L.L.P.P.E.P.R. Look, Lift, Push, Pull, Educate, Prescribe, Review ”
Q. Can you share a dry eye case study that illustrates some of the key points you have mentioned here?
A case that springs to mind is Gwen,* a 76-year-old woman with epiphora. Her chief concern was watery eyes that she had lived with for several years.
She had seen several eye care practitioners who had prescribed different eye drops, none of which had helped.
Major Findings
• Left meniscus L>R,
• Excess debris on lids and at base of lashes,
• Residual mascara on lids and lashes, (she had last used mascara two weeks prior),
• Incomplete blinking was severe; >45%,
• Reduced blink rate,
• Left inferior puncta showed some stenosis (slit form),
• Left ectropion,
• Inferior staining R>L,
I observed the patient wiping her eyes by pulling the eyelids down.
Management and Treatment
I instructed Gwen to:
• Use Zocular Foam at home twice daily,
• Cease eye wiping, change to dabbing,
• Practise blinking according to instruction, and
• Apply Cationorm for her right eye.
I advised that in my view, this treatment was unlikely to make a significant difference to Gwen’s epiphora and booked her in for a Blephasteam and mastrota expression at review. Additionally, I offered a referral for surgery if the treatment was inadequate.
Review Outcomes
When I reviewed Gwen two weeks later, her lids and lashes were clean and her meibomian glands were clear and easy to express.
Gwen reported total resolution of her epiphora since starting Zocular Foam and described it as “magical”.
Gwen’s OSDI score was two (normal). It would have been zero, however wind had caused irritation during the week.
*Patient name changed for anonymity.
Dr Margaret Lam is the National President of Optometry Australia. She practises optometry at 1001 Optical in Bondi Junction in Sydney, and teaches at the School of Optometry at The University of New South Wales as an Adjunct Senior Lecturer.
Martin Robinson graduated as an optometrist from Queensland University of Technology in 1994 and has owned Martins Eyecare, an independent optometry practice in Melbourne, since 2009. Mr Robinson has a keen interest in contact lenses and dry eye disease. He educates on dry eye disease, manages referred dry eye patients in practice, and works closely with ophthalmologists and oncologists helping diagnose and treat their dry eye patients. Since 2017 he has been Tasmanian State President of the Cornea and Contact Lens Society of Australia (CCLSA), and in 2022 he was elected as National President of the CCLSA.
References
1. Moreker, M.R., Thakre, N., Patel, R.C., Insulin eye drops for neurotrophic keratitis. Indian J Ophthalmol. 2023 Jul;71(7):2911–2912. DOI: 10.4103/IJO.IJO_872_23.
2. Cloudbreak Pharma, CBT-008 (webpage), available at: cloudbreakpharma.com/cbt-008 [accessed Feb 2024].
3. Watson, S.L., Jones, L.W., Rafaeli, O., DePuy, V., Celestial Study Group. Efficacy and safety of AZR-MD-001 selenium sulfide ophthalmic ointment in adults with meibomian gland dysfunction: A vehicle-controlled, randomized clinical trial. Ocul Surf. 2023 Jul;29:537–546. DOI: 10.1016/j.jtos.203.07.02.
4. Aldeyra, Allergic conjunctivitis (webpage), available at: aldeyra.com/pipeline-disease-areas/ocular-diseases/allergic-conjunctivitis/ [accessed Feb 2024].
5. Yeu, E., Wirta, D.L., Karpecki, P., Baba, S.N., Holdbrook, M., Saturn I Study Group. Lotilaner ophthalmic solution, 0.25%, for the treatment of demodex blepharitis: results of a prospective, randomized, vehicle-controlled, double-masked, pivotal trial (Saturn-1). Cornea. 2023 Apr 1;42(4):435-443. DOI: 10.1097/ICO.0000000000003097.
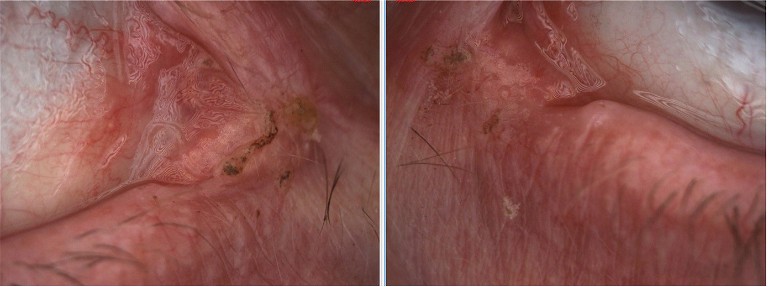
Figure 1. Photos from slit lamp illustrating epiphora; A) right eye and B) left eye.
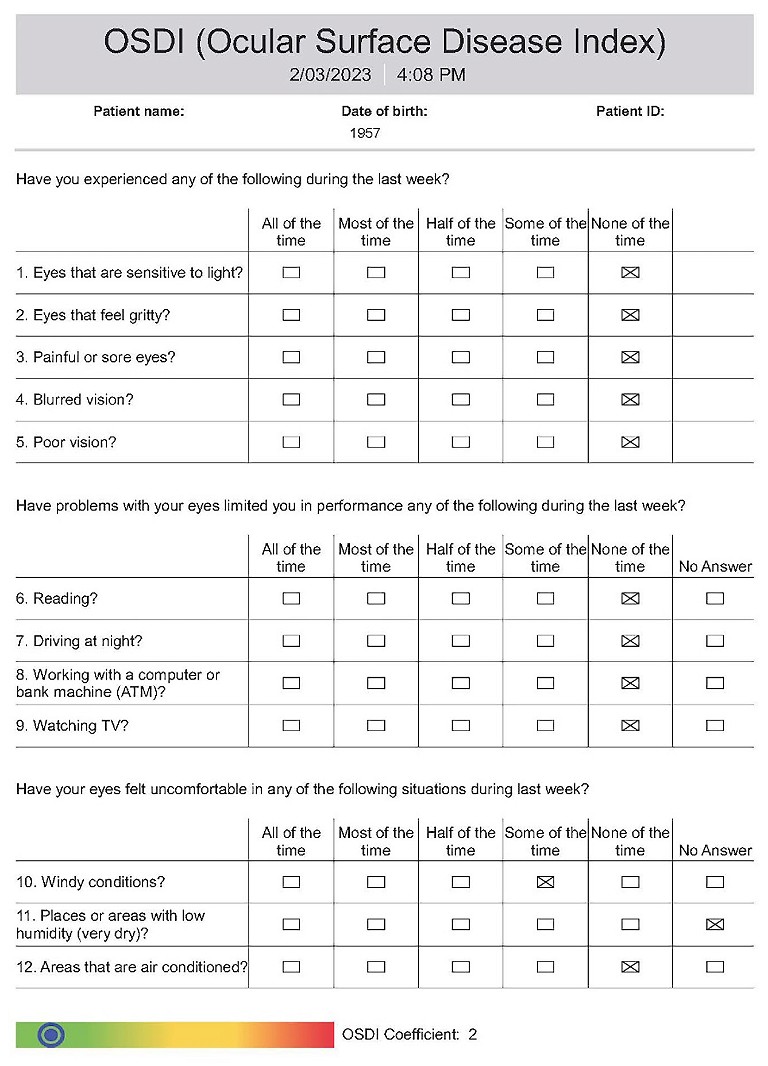
Figure 2. Ocular surface disease screening questionnaire.
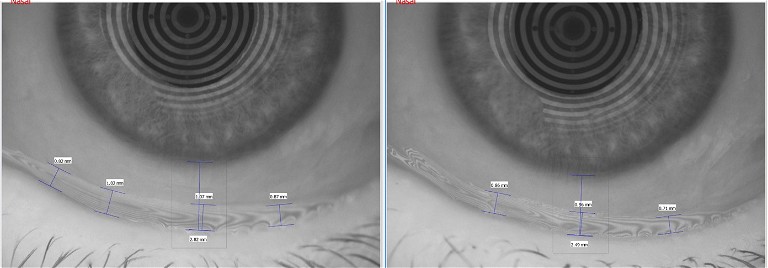
Figure 3. A) Before treatment, the patient’s tear meniscus ranged from 0.82–1.07 mm; and the distance from limbus to lid margin was 2.82 mm. B) At review, the patient’s tear meniscus ranged from 0.71–0.92 mm and distance from limbus to lid margin was 2.49 mm.