miophthalmology
Syndromic Myopia:
The Hidden Danger
WRITER Dr Loren Rose
The growing epidemic of myopia and, with it, the prevalence of high myopia1,2 (defined as greater than -6D or 26 mm in axial length3 ) has prepared us to expect the presentation of young children with high levels of myopia in our practice. However, as Dr Loren Rose writes, this group includes a small but very important subset of patients: those with syndromic myopia.
Syndromic myopia refers to the presentation of myopia (usually high and in the very young) where the myopia is only part of a broader condition.4
The group of diseases includes systemic genetic disorders affecting the body’s connective tissue synthesis. Collagen and elastin are structural components of the eye, as well as integral parts of our skin, cardiovascular, and musculoskeletal systems. Therefore, some of these syndromes can be life-threatening as well as sight-threatening.
Another systemic group of diseases that can present with myopia (often high myopia) is retinal dystrophies. We now know that the control of ocular growth is local in the peripheral retina. This internal regulation can be lost if there is an inherited dystrophy in the retinal cell layer. In fact, retinal dystrophy genes are highly associated with genes for abnormal refractive errors.5 This article will cover some of the most common presentations of syndromic myopia.
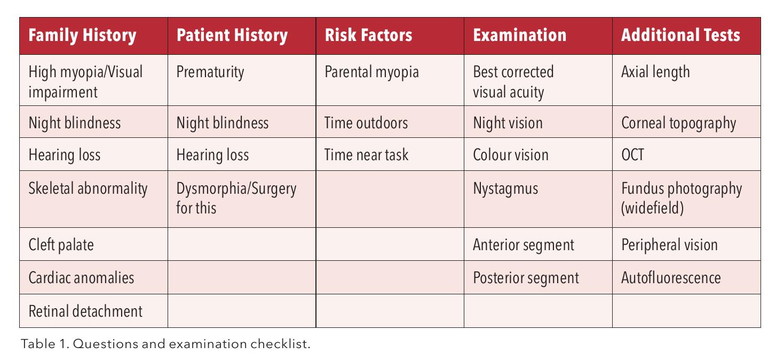
Retinal dystrophy and connective tissue diseases are often inherited and may have a family history; however, they can also occur de novo. Initial assessment with history taking may raise concerns, including a family history of poor vision at a young age, strong family history of retinal detachment, hearing defects, cleft palate, and sudden death due to aortic aneurysm or heart valve replacement. Specific questions regarding concerns with night, peripheral, and colour vision should be asked. Tests additional to standard slit lamp exam that are helpful, if available, include optical coherence tomography (OCT), fundus photography (wide field), and autofluorescence. I have developed a checklist (Table 1) of questions and possible additional clinic tests. This is worth having available as a standard to ensure best practice in distinguishing possible syndromic myopia.
Additional tools to raise our suspicion include the presence of ‘red flags’ during the consultation. Red flags have been described in the International Myopia Institute (IMI) white papers4 and should prompt a referral process to ensure an accurate diagnosis is made and any safety concerns are addressed. Of note, the red flags are a guide for referral and not an immediate cause for alarm for the parent and patient, as high myopia presents in the young on its own as well.
The first red flag is a child presenting with high myopia under 10 years of age.
The second red flag is if myopia in dioptres is greater than the age of the patient; for example, a child aged three presents with -4D.
The third red flag is poor vision with accurate refraction. This is a challenging one, as the child may often be initially undertreated due to the difficulty of accurate refraction or may be too young for accurate distance vision testing. Clues to poor vision and indications of referral include nystagmus and a history of poor night vision. Interestingly, children may not specifically complain of night vision problems, so it is useful to ask about a fear of the dark and also whether the child can navigate in the dark. Often, it is common for children to have a night light in their room so they may not experience the dark, as was the case in previous generations.

“The first red flag is a child presenting with high myopia under 10 years of age”
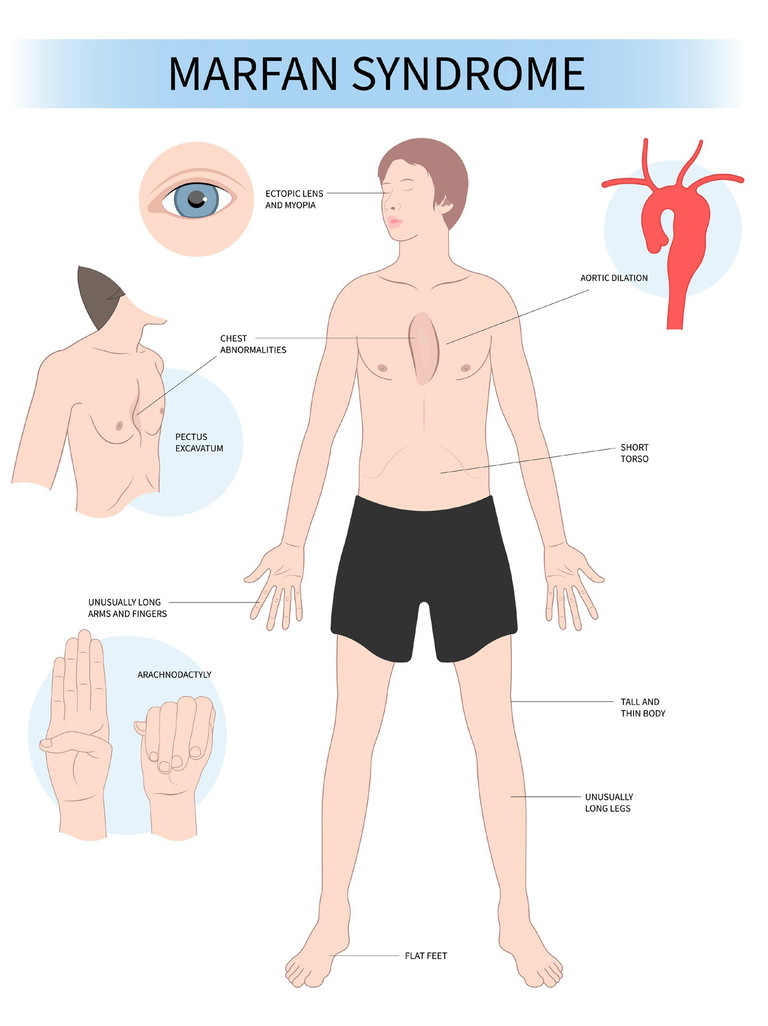
Figure 1. Signs of Marfan syndrome.
On examination, the fundus should be examined for pathology, although it should be noted that many retinal dystrophies do not have fundus pathology visible. The red flag prompts are useful reminders to refer the patient for additional testing, which may lead to further referral to a genetic service. If a syndrome is suspected, a referral to a paediatric ophthalmologist is recommended to confirm the findings and refer to a paediatric specialist for a systemic review and electrophysiology testing to examine retinal function. A paediatric review will include an examination of joints and listening for a heart murmur. Additional tests the paediatrician may order include a cardiac echo to ensure the cardiac blood vessels are not involved.
MARFAN SYNDROME
Marfan syndrome is caused by a defect in the FBN1 gene on chromosome 15 and is the most common of the inherited connective tissue disorders, with an estimated prevalence of approximately one in 10,000.6 It is an autosomal dominant (AD) condition, which means that a parent has a 50% chance of passing the defect to their child. However, up to 25% of cases can occur de novo.7
The ocular involvement includes myopia, a dislocated intraocular lens (ectopia lentis) due to zonular stretching, and retinal detachment.
The patient has a typical appearance, being tall and thin, with long legs and arms (arm span to height ratio > 1.05), as well as chest deformities. They are also at risk of a dilated aorta, which, if not treated with a valve replacement, can result in sudden death from an aortic dissection.
STICKLER SYNDROME
Stickler syndrome is an inherited disorder that affects collagen production, presenting with manifestations in the maxillofacial, ocular, auditory, and musculoskeletal systems.
There are four subtypes, including AD and autosomal recessive (AR) inheritance patterns, but they may also occur sporadically and exhibit a heterogeneous clinical presentation.7 Type 1 can present with facial features, including a cleft palate and a flat midface profile (Pierre-Robin syndrome, which includes a small jaw, Figure 2).
Otherwise, the only other feature visible is vitreous beading, which can be seen on the slit lamp with the patient dilated. Patients with Stickler syndrome are at high risk of retinal detachment even at a young age, and the most common cause of retinal detachment in children.7 As the condition also affects the vitreous, the natural support of the retina, which is solid in a child, is lost. Sadly, this condition can result in giant retinal detachment that is difficult to reattach, even with moderate amounts of myopia.
CONGENITAL STATIONARY NIGHT BLINDNESS
Congenital stationary night blindness (CSNB) is a retinal dystrophy that affects the processing within rod photoreceptors, specifically the retinoid recycling in the retinal pigment epithelium and the signal transmission via bipolar cells. There are 17 genes with more than 360 mutations and 670 alleles known to have an inheritance pattern of X-linked (most common), AD, and AR.8
Electrophysiology can serve as a diagnostic tool, with distinct measurement patterns observed for each mutation.8 Aspects of a patient history that may raise concerns include poor night vision or dim-light vision and, rarely, poor colour vision. The examination would reveal mild to moderate suboptimal vision, typically around a bestcorrected visual acuity of 6/12, but can be severe. Nystagmus may also be present; however, a normal fundus examination is often observed. This retinal dystrophy is non-progressive, so vision should be stable with accurate refraction adjustments for progressive myopia.
RETINOPATHY OF PREMATURITY
A premature child with or without retinopathy of prematurity (ROP) is at risk of high myopia.4 When a child is born prematurely, they are at risk of underdeveloped retinal vascularity, which can manifest as abnormal vascularity and potentially lead to retinal detachment if left untreated.
Standard treatment has involved significant laser or cryotherapy ablation of ischemic retinal tissue to prevent retinal detachment in neonates. This treatment is associated with a significant risk of high myopia, which has a different growth pattern compared to general axial growth. Current advances in the treatment paradigm for ROP now include anti-VEGF injections instead of ablation, which may change the long-term prevalence of high myopia associated with ROP, although there is still an association of high myopia in this treatment group.9

Figure 2. Signs of, and medical interventions for, Pierre-Robin syndrome.
TREATMENT FOR PROGRESSIVE SYNDROMIC MYOPIA
All interventional studies aiming to prevent progressive myopia exclude syndromic patients from their treatment groups.
Therefore, none of the treatment trials are directly applicable to this patient group. This information must be conveyed and explained to the patient and family as part of the consent, if any treatment intervention is considered. It may be, especially in the case of retinal dystrophies, that the abnormal periphery may not respond to available interventions based on our current understanding of ocular growth regulation. Therefore, any positive effect on myopia control may depend on the extent of peripheral pathology present.
Additionally, any systemic associations should be taken into consideration. For example, although low-dose atropine is not considered to have systemic effects, we know it can be used systemically. For this reason, I would avoid it in a patient with cardiac associations such as Marfan syndrome.
Various types of syndromic myopia may exhibit different growth patterns compared to the general population. It is not uncommon that syndromic myopia presents with very high myopia at a young age with little progression after diagnosis.4 However, once the patient has been assessed for these systemic concerns and axial progression is documented, I do consider intervention for their progressive myopia, again with the consent of the patient and family. This approach has also been advocated in the IMI white paper, with consideration and individualised care tailored to their condition.4
My initial intervention is similar to the general patient group with peripheral defocus glasses. These are well-tolerated and have not been reported to cause a rebound in the general population, and so are often my first choice. However, if a patient has photophobia as a symptom of their retinal dystrophy, then peripheral defocus glasses and low-dose atropine may not be suitable. Additionally, as some of these conditions have abnormal best corrected visual acuity, it is paramount that visual correction is up-to-date and accurate.
SUMMARY
It is important to consider the possibility of syndromic myopia as a diagnosis with the presentation of high myopia in a young patient. Comprehensive history taking and examination, as per the checklist in Table 1, may suggest referral to a paediatric ophthalmologist for further testing and diagnosis. Additionally, ‘red flags’ can aid in raising suspicion, with a referral recommended if the child has higher myopia in dioptres than their age or if they have high myopia and are younger than 10 years of age.
Treatment, if they have progressive myopia and have been medically cleared, can be considered as an individualised approach, ensuring the patient and family understand that there is no evidence that the intervention tools apply to their condition.
To earn your CPD hours from this article, visit mieducation.com/syndromic-myopia-thehidden-danger.
Dr Loren Rose BSc (Hons I) MBBS (Hons) PhD FRANZCO completed her medical degree from the University of Sydney, graduating with MBBS (Honours). Prior to that, she completed a Bachelor of Science from the University of Sydney, graduating with Honours (Class I) in Visual Neuroscience.
Dr Rose completed her ophthalmic training at the Royal Eye and Ear Hospital in Victoria. Following this, she underwent a fellowship in paediatric ophthalmology at the Royal Children’s Hospital, Melbourne. Now based in Sydney, she is a clinical senior lecturer at Macquarie University, and she practises privately at Sydney Eyecare Burwood, Sydney. In 2021, she completed her PhD, titled Myopia in Children, at Macquarie University.
References
1. Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036-42. doi: 10.1016/j.ophtha.2016.01.006.
2. Wolffsohn JS, Whayeb Y, Logan NS, et al. IMI - Global trends in myopia management attitudes and strategies in clinical practice -2022 update. Invest Ophthalmol Vis Sci. 2023;64(6):6. doi: 10.1167/iovs.64.6.6.
3. Flitcroft DI, He M, Jonas JB, et al. IMI - Defining and classifying myopia: A proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci 2019;60(3):M20-M30. doi: 10.1167/iovs.18-25957.
4. Flitcroft I, Ainsworth J, Chia A, et al. IMI - Management and investigation of high myopia in infants and young children. Invest Ophthalmol Vis Sci. 2023;64(6):3. doi: 10.1167/iovs.64.6.3.
5. Tedja MS, Haarman AEG, Meester-Smoor MA, et al. IMI - Myopia genetics report. Invest Ophthalmol Vis Sci. 2019;60(3):M89-M105. doi: 10.1167/iovs.18-25965.
6. Chiu HH, Wu MH, Chen HC, et al. Epidemiological profile of Marfan syndrome in a general population: a national database study. Mayo Clin Proc. 2014;89(1):34-42. doi: 10.1016/j.mayocp.2013.08.022.
7. Salles Rosa Neto N, Pereira IA, Sztajnbok FR, et al. Unraveling the genetic collagen connection: clinical and therapeutic insights on genetic connective tissue disorders. Adv Rheumatol. 2024;64(1):32. doi: 10.1186/s42358-024-00373-z. [published online first: 20240425]
8. Durajczyk M, Lubinski W. Congenital stationary night blindness (CSNB) case reports and review of current knowledge. J Clin Med. 2025;14(4) doi: 10.3390/ jcm14041238. [published online first: 20250213].
9. Crouch ER, Kraker RT, Wallace DK, et al. Secondary 12-Month ocular outcomes of a Phase 1 dosing study of bevacizumab for retinopathy of prematurity. JAMA Ophthalmol. 2020;138(1):14-20. doi: 10.1001/ jamaophthalmol.2019.4488.