mieyecare
Infectious Keratitis
The Potential in Novel Therapies
WRITER Dr Sanjay Marasini
Infectious keratitis can have severe consequences, often leading to permanent vision loss. These infections are typically treated with targeted antibiotic therapy, which is usually effective. However, the growing global issue of antibiotic resistance has raised concerns about the effectiveness of antibiotics against ocular isolates and the potential for suboptimal treatment outcomes. As new classes of antibiotics remain scarce, there is an increasing need for alternative antimicrobial treatments. This article explores a novel lightbased anti-infective technology currently being investigated as a potential solution for managing infectious keratitis.
INFECTIOUS KERATITIS: A GLOBAL BURDEN
Infectious keratitis is an infection of the cornea caused by various pathogens, including bacteria, fungi, protozoa, and viruses. It typically presents with a corneal epithelial defect, corneal infiltrates, and an anterior chamber reaction, often accompanied by severe pain. If left untreated, infectious keratitis can lead to vision loss or require surgical intervention. Although it is relatively rare in developed countries, it remains a significant health concern in lowincome countries, where it is considered a silent epidemic.1
In Australia, recent data indicate that up to 60% of patients with infectious keratitis require at least seven days of hospital admission.1 This prolonged hospitalisation contributes to high individual and systemic healthcare costs, with treatment estimated at AU$8,013 per patient, and the total annual cost of care estimated at $13.58 million. Globally, infectious keratitis is the fifth leading cause of blindness.2 The incidence of the condition varies by population, with rates ranging from 0.5 to 79.9 cases per 10,000 individuals annually, and higher rates observed in low-income countries. The disease is responsible for more than two million cases of unilateral blindness worldwide each year.3
While antibiotics are typically effective against infectious keratitis, several factors contribute to suboptimal treatment outcomes and increased medical costs. These include the rising global trend of antibiotic resistance, the limited range of available antimicrobial drugs, lengthy diagnostic processes, and the presence of polymicrobial infections. Additionally, the development of new broadspectrum antimicrobial agents has been slow, with new compounds often taking years to reach clinical use. Consequently, there is a growing demand for non-pharmacological approaches to manage corneal infections, which may help circumvent the challenges posed by antimicrobial resistance.
One promising strategy is antimicrobial photodynamic therapy, including corneal collagen crosslinking, which has shown effectiveness in treating severe, non-responsive
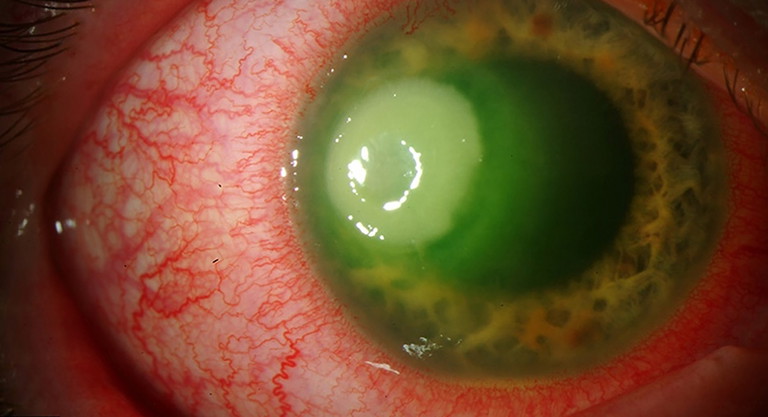
Figure 1. Pseudomonas keratitis in a 34-year-old male with a history of contact lens wear. The image was adapted from Eyerounds.org under Creative Commons noncommercial licence.
cases of corneal infections by targeting a wide range of pathogens. Other light-based therapies, either on their own, for example, antimicrobial blue light (400–470 nm), or combined with photosensitisers such as red light (660 nm) with methylene blue, red light (633 nm) with toluidine blue O, green light (500–551 nm) with rose bengal, as well as phage therapy and probiotics, are also under investigation.4 Some of these approaches have reached clinical trial stages, and a growing body of preclinical and limited clinical studies supports their efficacy.
This article focusses specifically on the evidence surrounding the potential of ultraviolet C (UVC) light in managing corneal infections.
UNDERSTANDING CORNEAL INFECTIONS
The healthy ocular surface possesses strong defences against microbial pathogens. Anatomical barriers – including the eyelids, eyelashes, tear film, and corneal epithelium – prevent microbial invasion of the cornea.5 Additionally, chemical barriers formed by tear proteins such as lactoferrin, defensins, secretory IgA, and lipocalin A inhibit bacterial growth, adherence, and survival.6 When these defence mechanisms are compromised, the cornea becomes more vulnerable to infiltrative events and infections.
Several factors predispose individuals to infectious keratitis, including contact lens wear, trauma, ocular surface disease, corneal surgery, and systemic conditions such as diabetes.7 Due to overlapping signs and symptoms, infectious keratitis is often underdiagnosed and can be misdiagnosed, potentially leading to worsening of the condition if inappropriate antimicrobial agents or corticosteroids are prescribed.8
Infectious keratitis is typically classified according to the causative microorganism into bacterial, fungal, viral, and parasitic aetiologies (Figure 1). The incidence of these infections varies globally, influenced by factors such as geographic location, climate, socioeconomic conditions, and seasonal variations.7 Bacterial keratitis remains the most prevalent form worldwide. However, viral keratitis, particularly that caused by herpes simplex virus, and Acanthamoeba keratitis, although less common, represent significant causes of corneal blindness in developed countries.9 In contrast, bacterial and fungal keratitis are more frequently encountered in low-income countries, where they are often associated with increased risk factors such as ocular trauma (especially from agricultural injuries), limited access to healthcare, lower socioeconomic status, and adverse environmental conditions.7
A wide range of microorganisms can be pathogenic to the cornea, but broadspectrum antibiotics, which have been commonly used since the early 1900s, have gradually become less effective. Globally, the preferred initial treatment for bacterial keratitis typically involves antibiotics without corticosteroids. This often includes either dual therapy, which combines a fortified cephalosporin (e.g., cefuroxime 5%) and an aminoglycoside (e.g., tobramycin 1.35%), or monotherapy with a topical fluoroquinolone (e.g., ciprofloxacin 0.3% or ofloxacin 0.3%). In Australia, topical monotherapy is prescribed for up to 80% of bacterial corneal infections.10
The literature indicates a decreasing sensitivity of bacterial isolates to ciprofloxacin and newer generations of fluoroquinolones, with resistance rates ranging from 1% to 36%.11-13 While inconsistencies in findings are noted, potentially due to country-specific prescribing practices, overall trends suggest there is increasing resistance of bacterial keratitis isolates to potent antibiotics. These antibiotics, which were a major line of defence against bacterial keratitis just a few decades ago, now have reduced effectiveness.
UVC LIGHT: MECHANISM OF ACTION
Ultraviolet (UV) radiation, spanning the electromagnetic spectrum from 100 nm to 400 nm, is used in various clinical applications, particularly in ophthalmology. Examples include excimer laser photorefractive keratectomy (193 nm) and corneal collagen crosslinking (365 nm). UV light is classified into three categories based on wavelength: UVC (200–280 nm), UVB (280–320 nm), and UVA (320–400 nm), with only UVA and UVB reaching the Earth’s surface. UVC, often called “germicidal UV”, is a potent antimicrobial agent that is highly effective against a broad range of pathogens, including multidrug-resistant bacteria. Due to its shorter wavelength, UVC has higher energy, with photons at 265 nm containing 1.37 times more energy than UVA (365 nm) and 1.12 times more energy than UVB (300 nm).14 This reactivity allows UVC to break apart oxygen molecules (O2 ), creating atomic oxygen, which then combines with O2 to form ozone (O3 ). As a result, UVC is absorbed by the stratosphere, and the UV light reaching the Earth’s surface consists of no UVC, about 7.3% UVB, and up to 25.5% UVA.15
“There is a growing demand for nonpharmacological approaches to manage corneal infections, which may help circumvent the challenges posed by antimicrobial resistance”
UVC kills microorganisms by damaging their DNA and disrupting cellular homeostasis. This interaction generates DNA photoproducts, such as cyclobutane pyrimidine dimers,16 which can lead to immediate cell death or impair replication. Unlike UVC and UVB, UVA is weakly absorbed by DNA but can interact with other cellular chromophores, inducing oxidative changes and generating highly energetic and reactive free oxygen species. These species induce covalent bonding between molecules while also killing all types of microorganisms, including bacteria, yeast, and fungi.17 As a result, while UVC and UVB can directly kill microorganisms (UVB being more mutagenic than UVC), UVA requires a photosensitising substrate, such as riboflavin, to enhance its antimicrobial efficacy. This property of UVA is harnessed in corneal collagen crosslinking that’s primarily used in ophthalmology to impede the progression of keratoconus. Corneal collagen crosslinking is used to alter the biomechanical behaviour of the cornea to treat keratoconus and currently forms a standard procedure in slowing keratoconus progression. It involves applying riboflavin eye drops (0.5% riboflavin/0.1% dextran solution) to the cornea, followed by UVA illumination at an irradiance of 3.0 mW/cm² to deliver a total dose of 5.4 J/cm².18 The procedure is time consuming and invasive, as it may also require the removal of the corneal epithelium to ensure adequate riboflavin penetration, leading to discomfort and pain until the epithelium heals.19
To address challenges in corneal collagen crosslinking, an accelerated protocol has been developed that reduces UVA exposure time while maintaining the same energy dose (Bunsen-Roscoe law of reciprocity). However, corneal collagen crosslinking remains invasive and carries risks, such as secondary infection and potential damage to deeper ocular structures, including the corneal endothelium. It is also not suitable for thin corneas (<400 µm), and the need for a photosensitising substrate introduces additional risks, such as possible damage to limbal stem cells.20 In contrast, UVC does not require a secondary substrate, acting independently to achieve its antimicrobial effect.
UVC EVIDENCE FROM PRECLINICAL STUDIES
Preclinical studies have demonstrated potential for antimicrobial UVC in being effective in treating infectious keratitis.21 UVC exposure targets a wide range of bacterial and fungal pathogens, including antibiotic-resistant strains. In agar models, five seconds of UVC exposure was sufficient, while in a murine model 15 seconds of exposure was required. When administered in two doses, four hours apart, UVC successfully managed Pseudomonas keratitis within 24 hours, with complete re-epithelialisation observed in most cases. Additionally, UVC has shown promise in inhibiting bacterial biofilms.22
Given its broad antimicrobial activity, UVC has the potential to serve as a valuable empiric treatment for corneal infections, especially when the causative pathogen is unknown. In cases of corneal ulcers, where diagnostic results are pending, UVC could help control infection while awaiting laboratory identification. This approach would be particularly beneficial when diagnostic procedures introduce delays in receiving targeted treatment. UVC could potentially be applied immediately after tissue debridement and sample collection, providing early reduction in microbial load. Prompt UVC application at the site of infection may thus improve patient outcomes, and debriding the corneal epithelium would enhance UVC penetration into deeper corneal layers, as the epithelium typically acts as a barrier.
Our studies show that therapeutic UVC doses at 265 nm, effective in treating murine models of Pseudomonas corneal infections, appear safe to the DNA.23 Safety was assessed by examining the formation of cyclobutane pyrimidine dimers in murine corneas and human cultured corneal epithelial cells. Recent findings suggest that the safety margin for UVC treatment of corneal infections is wider than previously recognised, based on studies of corneal depth penetration, which would indicate preservation of limbal stem cells. UVC is rapidly absorbed by the cornea due to its shorter wavelength, preventing it from reaching deeper structures where the limbal stem cells are located.24 High UVC doses, well beyond that of a clinical dose (five-minute exposure), appear to affect only the surface epithelium, which naturally undergoes normal desquamation every seven to 10 days, thus minimising the chances of carrying the defect in the long term.
“Preclinical studies show that UVC can effectively target pathogens while minimising damage to surrounding tissues”
Ongoing studies are investigating the effects of UVC on corneal proteins, nerves, and keratocytes. First-in-human studies are set to begin in New Zealand to evaluate the safety and efficacy of UVC application to the human cornea with a view to treating corneal infection in later studies. UVC can be directed precisely toward the site of infection, enabling targeted exposure of pathogens, with limited UV light exposure. This targeted approach may reduce the need for such frequent or prolonged antibiotic use, or potentially eliminate the need for antibiotics, for example, where prophylactic antibiotic cover is desired. While existing studies demonstrate the safety and efficacy of UVC applied therapeutically for corneal infections, additional research is needed to assess patient tolerance and confirm the safety of the low dose UVC applied in these treatments.
FUTURE DIRECTIONS
Ongoing research is steadily building evidence supporting the safety and efficacy of low-intensity UVC in treating corneal infections in preclinical models, with human trials due to commence in the near future. As antibiotic resistance continues to rise, UVC shows promise as a supplementary treatment, most likely for use in combination with other therapies, for localised superficial corneal infections. Its broad-spectrum effectiveness against bacteria, fungi, and viruses – regardless of antimicrobial resistance – positions UVC as a potential first-line treatment for corneal infections. While the preclinical results are encouraging, further human trials are essential to confirm these findings and establish UVC as a safe, reliable and effective therapeutic approach.
CONCLUSION
Corneal infections can progress rapidly, leading to severe outcomes such as permanent blindness, making timely and effective treatment crucial. Although antibiotics are typically effective, the growing issue of antibiotic resistance highlights the need for alternative antimicrobial therapies. Lightbased treatments, particularly UVC, offer promising solutions, as they are effective against a wide range of microorganisms.
Preclinical studies show that UVC can effectively target pathogens while minimising damage to surrounding tissues, including those housing limbal stem cells. The ability to reduce microbial load for a wide range of pathogens, including bacterial, yeast, fungal, and viral, highlights UVC’s possible empiric role, which could potentially be accompanied by reduced dependence on current antimicrobials.
Further pre-clinical and clinical research is warranted in continuing to explore this exciting new area, but UVC’s promising safety profile may see UVC become a valuable tool in rapidly and effectively helping manage corneal infection.

Dr Sanjay Marasini BOptom PhD is a Senior Research Fellow with the Health Research Council of New Zealand, where he leads innovative projects on nonpharmacological anti-infective technologies, including light-based therapies and probiotics. He works in the ocular surface laboratory directed by Professor Jennifer Craig at the University of Auckland.
Dr Marasini’s research expertise lies in translational biomedical science, focussing on advanced, non-pharmacological approaches to infection management.
References
1. Stapleton F. The epidemiology of infectious keratitis. Ocul Surf. 2023 Apr;28:351-363. doi: 10.1016/j. jtos.2021.08.007.
2. Daley JR, Lee MK, Samarawickrama C, et al. Epidemiology and economic cost analysis of microbial keratitis from a tertiary referral hospital in Australia. Pathogens. 2023 Mar 5;12(3):413. doi: 10.3390/ pathogens12030413.
3. Flaxman SR, Bourne RRA, Taylor HR, et al. Vision Loss Expert Group of the Global Burden of Disease Study. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017 Dec;5(12):e1221-e1234. doi: 10.1016/S2214-109X(17)30393-5.
4. Ung L, Bispo PJM, Chodosh J, et al. The persistent dilemma of microbial keratitis: Global burden, diagnosis, and antimicrobial resistance. Surv Ophthalmol. 2019 May-Jun;64(3):255-271. doi: 10.1016/j. survophthal.2018.12.003.
5. Marasini S, Craig JP, Dean SJ, Leanse LG. Managing corneal infections: Out with the old, in with the new? Antibiotics (Basel). 2023 Aug 18;12(8):1334. doi: 10.3390/ antibiotics12081334.
6. Evans DJ, Fleiszig SM. Why does the healthy cornea resist Pseudomonas aeruginosa infection? Am J Ophthalmol. 2013 Jun;155(6):961-970.e2. doi: 10.1016/j. ajo.2013.03.001.
7. Palomar APD, Montolío A, Bose T, et al. The innate immune cell profile of the cornea predicts the onset of ocular surface inflammatory disorders. J Clin Med. 2019 Dec 2;8(12):2110. doi: 10.3390/jcm8122110.
8. Hirano K, Tanaka H, Kato K, Araki-Sasaki K. Topical corticosteroids for infectious keratitis before culture-proven diagnosis. Clin Ophthalmol. 2021 Feb 16;15:609-616. doi: 10.2147/OPTH.S297202.
9. Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012 Sep;57(5):448-62. doi: 10.1016/j. survophthal.2012.01.005.
10. Keay L, Edwards K, Stapleton F, et al. Microbial keratitis predisposing factors and morbidity. Ophthalmology. 2006 Jan;113(1):109-16. doi: 10.1016/j.ophtha.2005.08.013.
11. Chalita MR, Höfling-Lima AL, Belfort R Jr, et al. Shifting trends in in vitro antibiotic susceptibilities for common ocular isolates during a period of 15 years. Am J Ophthalmol. 2004 Jan;137(1):43-51. doi: 10.1016/s00029394(03)00905-x.
12. Fong C-F, Hu F-R, Tseng C-H, Wang IJ, Chen W-L, Hou Y-C. Antibiotic susceptibility of bacterial isolates from bacterial keratitis cases in a university hospital in Taiwan. Am J Ophthalmol. 2007;144(5):682-9. doi: 10.1016/j. ajo.2007.06.038.
13. Alexandrakis G, Alfonso EC, Miller D. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology. 2000 Aug;107(8):1497-502. doi: 10.1016/s0161-6420(00)00179-2.
14. Marasini S. Exploring the potential of UVC in treating superficial corneal infections. Auckland: The University of Auckland; 2019. Available at: researchspace.auckland. ac.nz/items/0946fbc2-1667-415e-ac0c-59f95f3eb845/full [accessed April 2025].
15. Häder DP, Lebert M, McKenzie R, et al. ELDONET – a decade of monitoring solar radiation on five continents. Photochem Photobiol. 2007 Nov-Dec;83(6):1348-57. doi: 10.1111/j.1751-1097.2007.00168.x.
16. Anna B, Blazej Z, Andrzej S, et al. Mechanism of UV-related carcinogenesis and its contribution to nevi/ melanoma. Expert Rev Dermatol. 2007;2(4):451-469. doi: 10.1586/17469872.2.4.451.
17. Marasini S, Zhang AC, Craig JP, et al. Safety and efficacy of UV application for superficial infections in humans: A systematic review and meta-analysis. Ocul Surf. 2021 Jul;21:331-344. doi: 10.1016/j.jtos.2021.03.002.
18. Wong RL, Gangwani RA, Yu LW, Lai JS. New treatments for bacterial keratitis. J Ophthalmol. 2012;2012:831502. doi: 10.1155/2012/831502.
19. Spörl E, Huhle M, Kasper M, Seiler T. Increased rigidity of the cornea caused by intrastromal crosslinking. Der Ophthalmologe: Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 1997;94(12):902-6. German doi: 10.1007/s003470050219.
20. Snibson GR. Collagen cross-linking: a new treatment paradigm in corneal disease - a review. Clin Exp Ophthalmol. 2010 Mar;38(2):141-53. doi: 10.1111/j.14429071.2010.02228.x.
21. Marasini S, Dean SJ, Craig JP, et al. Preclinical confirmation of UVC efficacy in treating infectious keratitis. Ocul Surf. 2022 Jul;25:76-86. doi: 10.1016/j. jtos.2022.05.004.
22. Marasini S, Dean SJ, Craig JP, et al. In vitro anti-biofilm efficacy of therapeutic low dose 265 nm UVC. J Photochem Photobiol B. 2025 Feb;263:113091. doi: 10.1016/j. jphotobiol.2024.113091.
23. Marasini S, Mugisho OO, Craig JP, et al. Effect of therapeutic UVC on corneal DNA: Safety assessment for potential keratitis treatment. Ocul Surf. 2021 Apr;20:130138. doi: 10.1016/j.jtos.2021.02.005.
24. Marasini S, Dean S, Craig JP. Safety of therapeutic ultraviolet C light application at the limbus. Invest Ophthalmol Vis Sci. 2024;65(7):4121.