Rhegmatogenous Retinal Detachment: Updating Our Management Approach
Rhegmatogenous retinal detachment (RRD) is an ophthalmic emergency that requires urgent management to prevent or reduce permanent visual disability. This article aims to briefly review the basics of rhegmatogenous retinal detachments and, additionally, provide updates from recent studies by Dr Rajeev Muni and colleagues that have enhanced our understanding of the condition and improve the visual outcomes for our patients.
WRITERS Dr Tuan Tran, Dr Jessica Xiong, Dr Sebastian Waldstein, Professor Adrian Fung, Dr Isabela Martins Melo, and Dr Rajeev Muni.
LEARNING OBJECTIVES
On completion of this CPD activity, participants should:
1. Be aware that rhegmatogenous retinal detachment is an ophthalmic emergency requiring urgent management,
2. Understand the mechanism of RRD,
3. Recognise the signs and symptoms of a presenting RRD, and
4. Be aware of the latest evidencebased management options to optimise visual outcomes.
A rhegmatogenous retinal detachment develops when a full thickness retinal break (hole or tear) allows the accumulation of subretinal fluid (SRF) and separation of the neurosensory retina and retinal pigment epithelium (RPE).1 The normal apposition of the neurosensory retina and RPE is crucial for visual function. Thus, the separation of these layers and accumulation of subretinal fluid compromises vision and risks permanent vision loss if left untreated.1
The vast majority of retinal detachments occur in the acute setting, secondary to retinal breaks at the time of a posterior vitreous detachment (PVD),2 while 2.5–5% may be chronic RRDs caused by the gradual accumulation of SRF through asymptomatic atrophic holes without traction.3 These RRDs have been further described as dysregulated and regulated forms based on recent microstructural changes on optical coherence tomography (OCT),4 which will be discussed later in this article.
DEMOGRAPHICS
A RRD can occur at any age, but peak prevalence is 60–70 years with a male predilection. Studies have supported a bimodal distribution with the second most common age group peaking in 20–30 year olds, particularly in highly myopic patients.5,6 Overall, the incidence rate for RRD is approximately 1/10,000 per year,7 however there is an increasing annual incidence of 5.4/100,000 per decade.7 This is thought to be contributed by an ageing population, increased incidence of myopia, and earlier cataract surgery.8
CLINICAL EXAMINATION
The diagnosis of RRD is clinical. The key features on examination are to identify the location of all retinal breaks, establish the extent of the RRD, including whether the macula is involved, the extent of RRD in clock hours, presence or absence of a PVD, vitreous haemorrhage, and/or proliferative vitreoretinopathy (PVR), all of which play a pivotal role in determining the type of surgical intervention to undertake, and its timing.
Lincoff Rules have been well established over 40 years to help identify the location of primary causative retinal breaks in RRDs. Figure 1 outlines a summary of Lincoff Rules.9,10
MANAGEMENT OF RRD
The aims of RRD management are to achieve retinal reattachment, seal all breaks, and optimise visual outcomes. There are three main surgical interventions for management of RRDs: 1. pneumatic retinopexy (PnR), 2. scleral buckling (SB), and 3. pars plana vitrectomy (PPV). The choice of intervention is dependent on detachment location and extent, retinal tear location, presence of PVR, posturing compliance of the patient, lens status, the need for post-operative air flight, and surgeon preference.9 For macula sparing, small, localised acute RRDs, and chronic asymptomatic RRDs, demarcation with retinal laser photocoagulation alone can be effective as primary management (Figure 2).11
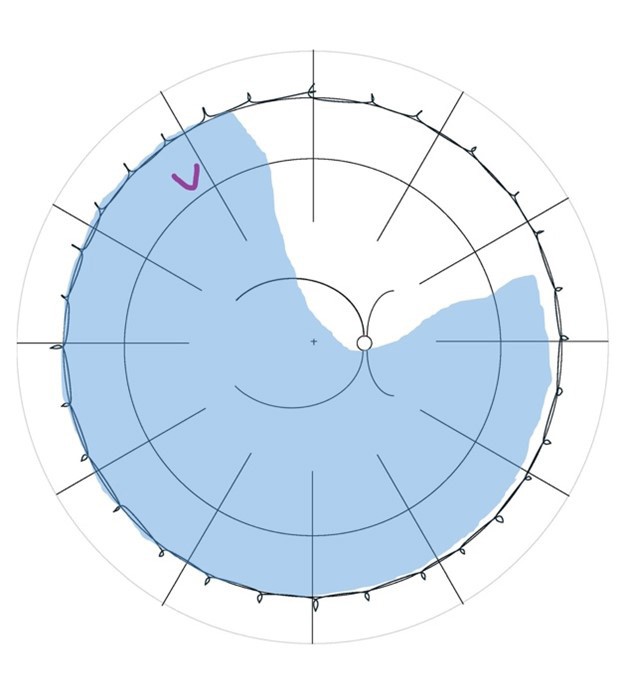
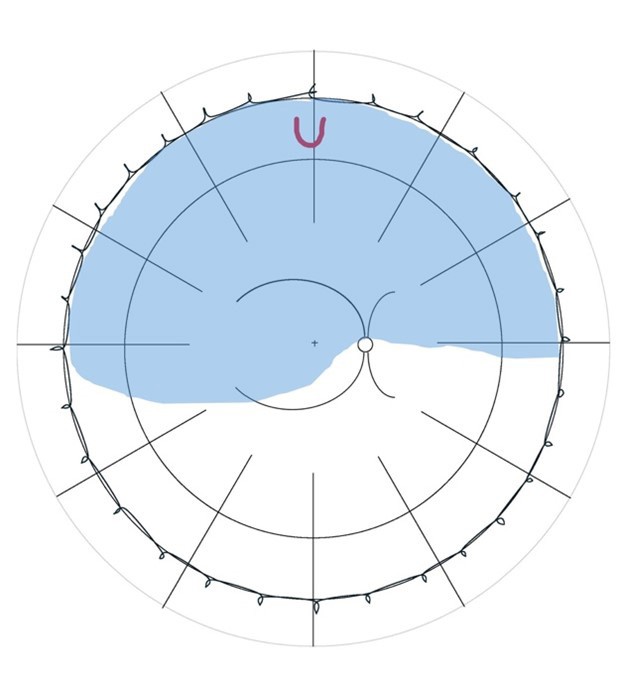
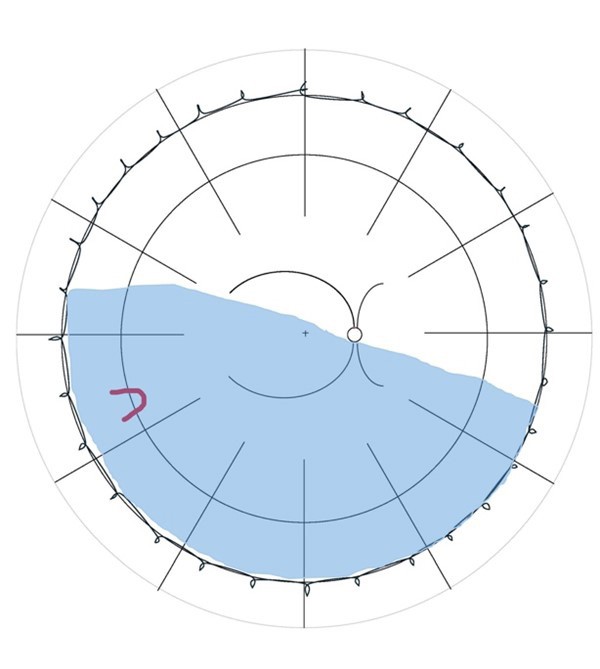
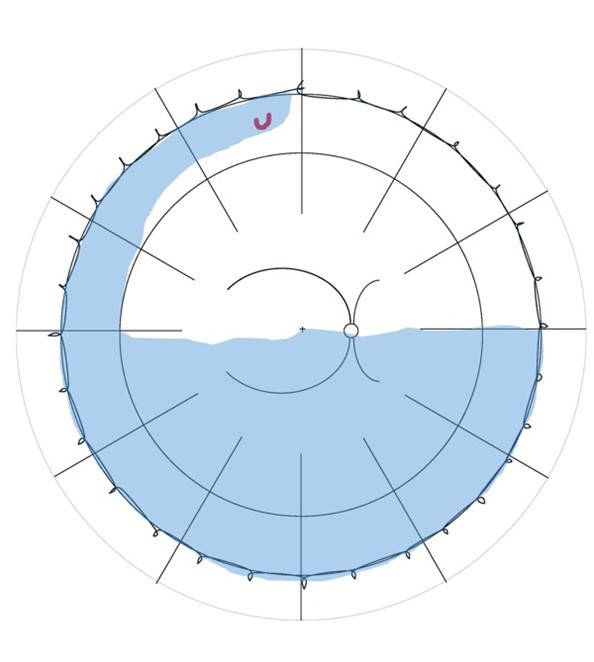
Figure 1. Summary of Lincoff Rules (blue = retinal detachment area and red = retinal break). A) Superior temporal or nasal RRDs, the primary break lies within 1.5 clock hours of the highest border. B) Total or superior RRDs that cross the 12 o’clock meridian, the primary break occurs at 12 o’clock or within 1.5 clock hours. C) Inferior RRDs, the higher side of the detachment contains the break. D) Inferior bullous RRDs, the primary break is usually a small hole close to 12 o’clock.
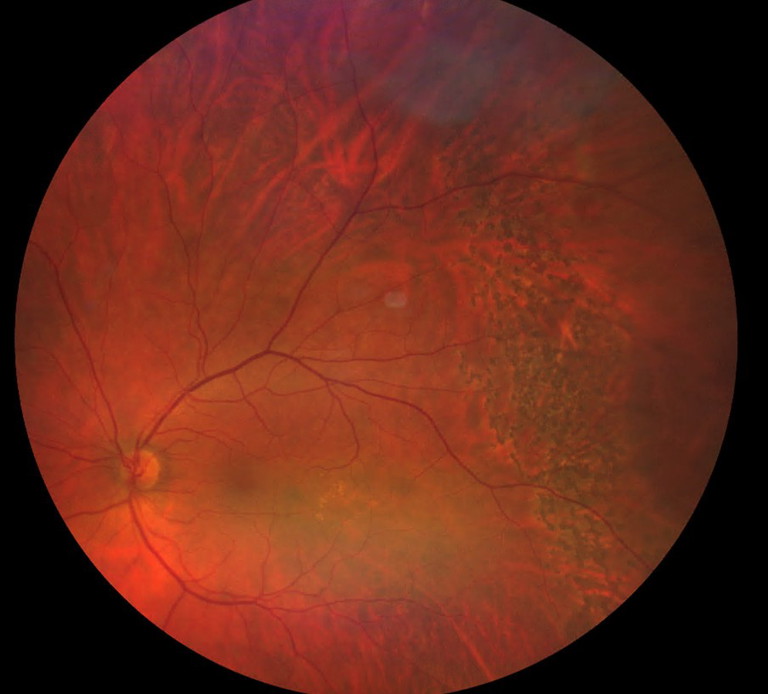
Figure 2. Left temporal macula sparing retinal detachment barricaded by retinal laser.
“The vast majority of retinal detachments occur in the acute setting, secondary to retinal breaks at the time of a posterior vitreous detachment”
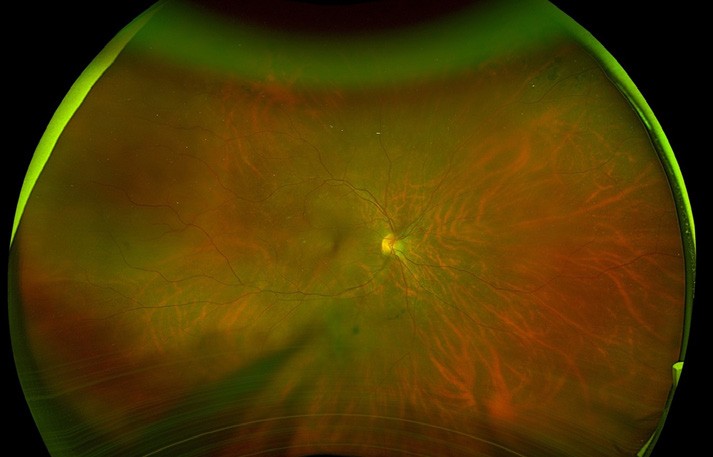
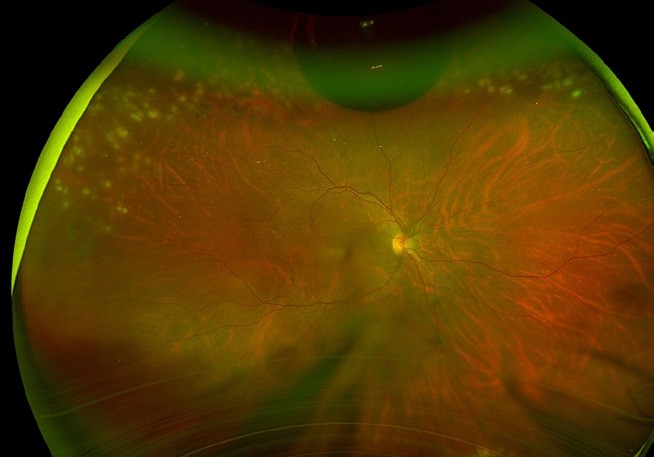
Figure 3. A) Right eye supertemporal macula on retinal detachment. B) Flattened retina with superior laser (white marks) and receding gas bubble five days post pneumatic retinopexy.
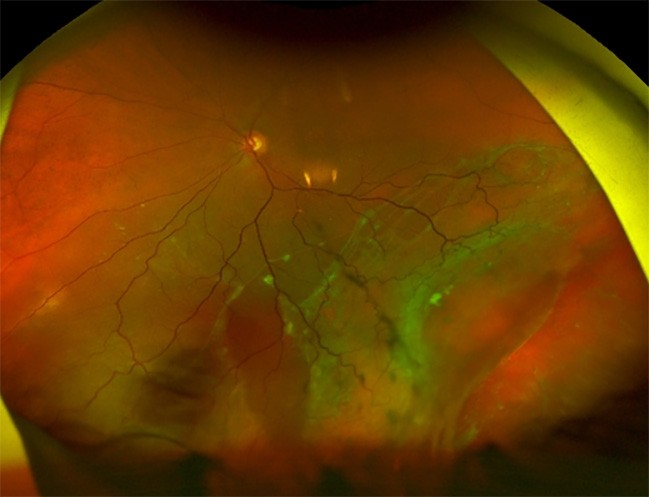
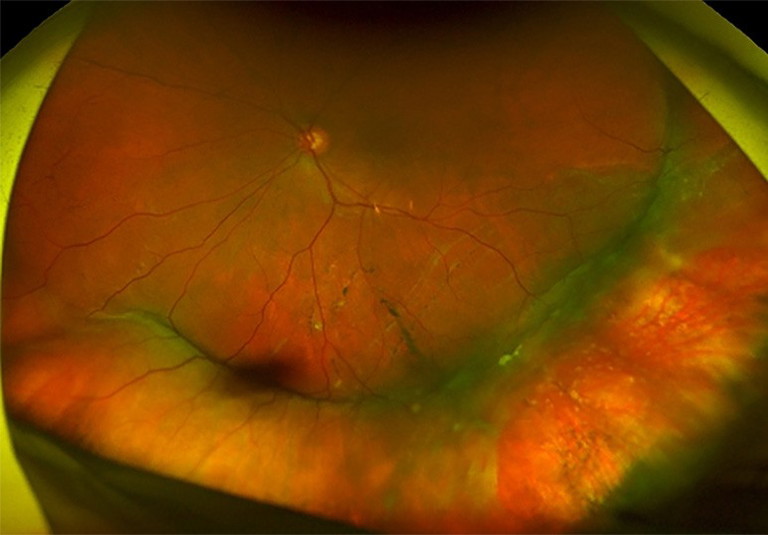
Figure 4. A) Left inferior and temporal chronic macula off retinal detachment with large inferotemporal break. B). Reattached retina post scleral buckle surgery with cryotherapy.
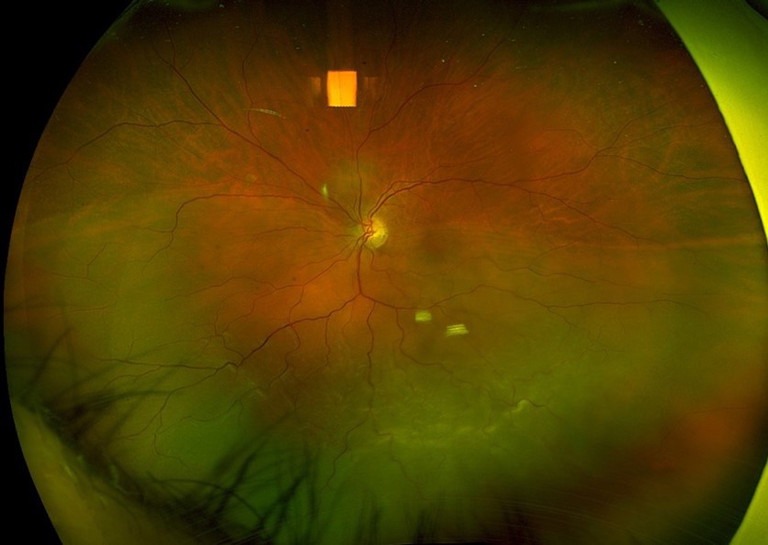
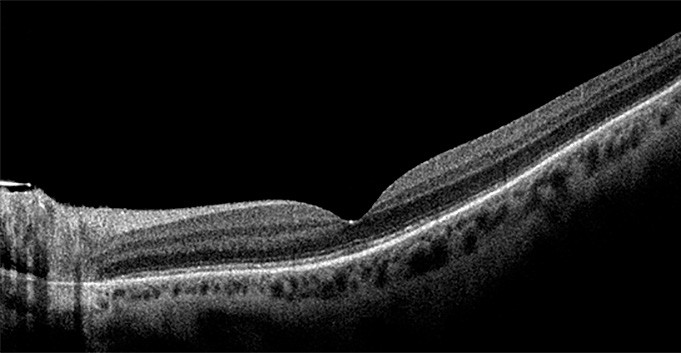
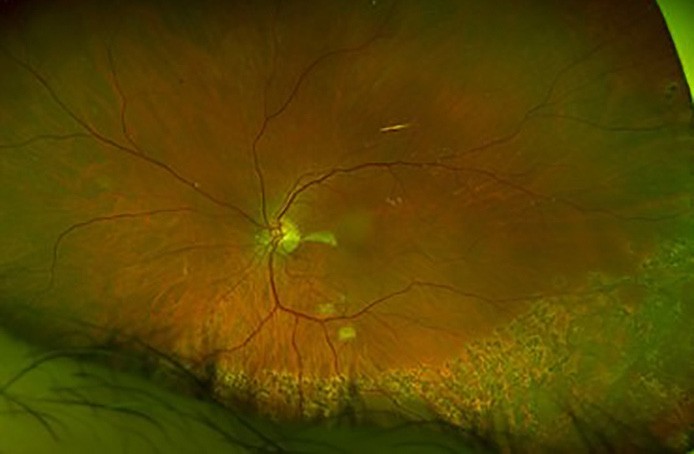
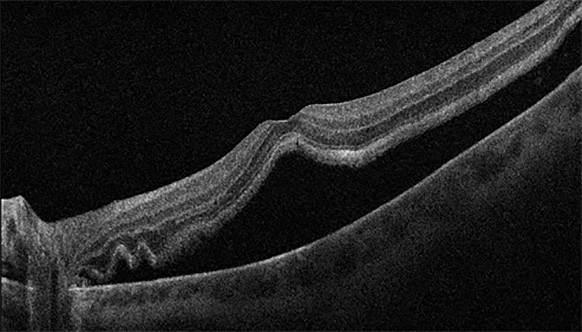
Figure 5. A) Left eye inferior retinal detachment involving the macula. B) OCT image demonstrating macular involvement in retinal detachment. C) Post vitrectomy surgery with retinal reattachment and silicone oil tamponade. D) OCT image demonstrating reattachment of macula post vitrectomy surgery.
Pneumatic Retinopexy
Treatment of RRD by PnR was first described by Dominguez in 1985.12 It entails injecting a small volume of expansile gas into the vitreous cavity and posturing the patient, such that the intravitreal gas tamponades the retinal break while the RPE pump reabsorbs the SRF. The retinal breaks are sealed with cryotherapy before, or laser retinopexy after the retina is reattached. PnR is suitable for detachments involving the superior retina with retinal breaks within one clock hour from eight o’clock to four o’clock, with minimal media opacity, no PVR, and in patients who are able to posture.13
More recently, one of the largest prospective randomised controlled trials in RRDs, the Pneumatic Retinopexy Versus Vitrectomy for the Management of Primary Rhegmatogenous Retinal Detachment Outcomes Randomized Trial (PIVOT) compared visual and anatomic outcomes in PnR versus PPV for management of primary RRD.14 The anatomic success was greater in the PPV group at 12 months (93.2% vs. 80.8%), however, visual acuity (VA) was superior in those undergoing PnR at every time point up to the end point of 12 months post intervention. The PnR group also demonstrated lower rates of vertical metamorphopsia and cataract at 12 months compared to the PPV group. Reduced vertical metamorphopsia is thought to be due to less retinal displacement after PnR compared with PPV. This supports PnR as an effective initial management option for primary RRDs that fit criteria, particularly in phakic patients. Further data from this trial have also demonstrated several benefits of PnR, such as better visionrelated quality of life, less retinal displacement and vertical metamorphopsia, less outerretinal folds, and photoreceptor disruption with improved visual acuity outcomes.
Scleral buckling, introduced in the 1950s, was the gold standard for RRD management for over 60 years.15 It is primarily performed on younger, myopic, phakic patients with a localised RRD, small anterior holes, or retinal dialyses without PVD or PVR. SB involves identifying the break, applying cryotherapy, and using a segmental or circumferential buckle to provide mechanical indentation and apposition of the retina and RPE. This allows the retinal break to close and SRF can be drained during surgery or left to spontaneously resorb by the RPE pump mechanism.
Scleral buckling is very successful as a primary repair technique for RRD, with success rates up to 95% at 20-year followup.16 However, with the rise of minimally invasive small gauge PPV and widefield intra-operative visualisation systems, SB has been less utilised in primary RRD repair. This may be due to SB being more timeconsuming and decreasing trainee exposure to the procedure.17 The Scleral Buckling Versus Primary Vitrectomy In Rhegmatogenous Retinal Detachment (SPR) study found phakic patients undergoing SB had a greater VA improvement than PPV, with less cataract progression. For pseudophakic patients, there was no VA difference between the two groups, but primary anatomical reattachment rate was significantly higher in the PPV group.18 In a recent meta-analysis comparing PPV with scleral buckling of 15,947 eyes, SB was found to have superior VA at last follow-up, however this was primarily due to phakic eyes developing cataracts post PPV.19 PPV was found to have higher risk of cataract and iatrogenic retinal breaks. There was no difference in primary and final anatomic reattachment rates.19 Overall, SB and PPV are considered comparable, with SB potentially being more favourable in patients who are phakic.18,20
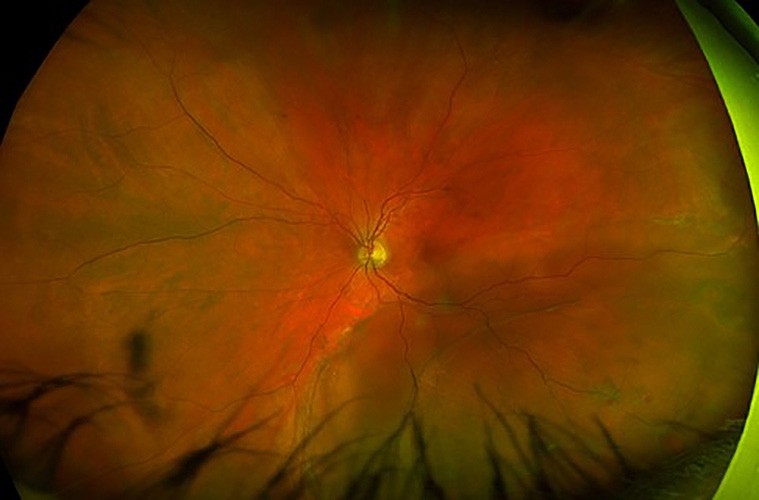
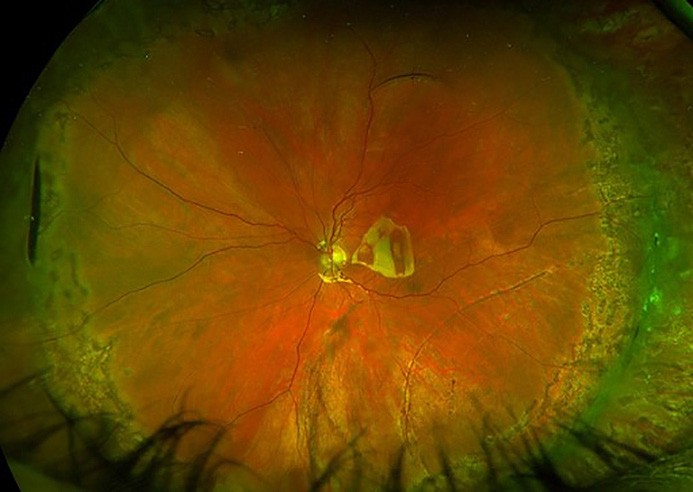
Figure 6. A) Left eye inferotemporal retinal detachment involving the macula. B) Retinal reattachment post combined scleral buckle and vitrectomy with encircling buckle indentation, 360° barrier laser, and silicone oil tamponade.
Pars Plana Vitrectomy
Pars plana vitrectomy has gained popularity since the 1970s, particularly with the advancement of ultra-widefield operating microscopes and micro-incisional (23 to 27 gauge) sutureless vitrectomy in the 2000s.21-23 Indications for PPV include those where SB is not appropriate, such as thin sclera, RRD with vitreous opacities limiting the retinal view, giant retinal tears, posterior breaks that cannot be reached with SB, presence of PVD, significant vitreoretinal traction that is unable to be relieved by SB, and the presence of PVR.9
Vitrectomy typically involves three transconjunctival sclerotomies to allow access for infusion fluid, illumination, and the vitrectomy cutter or other instruments into the eye. The vitrectomy cutter removes vitreous and relieves vitreoretinal traction around retinal tears, while PVR membranes can be peeled or removed from underneath the retina if present. SRF may then be drained through peripheral retinal breaks or a posterior retinotomy, with perfluorocarbon liquids (PFCL) sometimes used to aid flattening. Retinal tears are sealed with laser or cryotherapy, and the eye is filled with an intravitreal tamponade. Early positioning in the post-operative period is then adopted, depending on the location of the retinal detachment and type of tamponade.
Types of intravitreal tamponades for RRD include gas, oil, and heavy liquids. Of the gases, sulfur hexafluoride (SF6) is often used, particularly for smaller superior breaks in RRDs that are unlikely to be complicated by PVR. Silicone oil does not spontaneously reabsorb and requires an additional surgery for its removal at three to six months to minimise risk of retinal toxicity.24 The Silicone Oil study found the use of silicone oil tamponade resulted in superior VA and reattachment rate outcomes compared to SF6 but was equivalent to C3F8 in PVR RRDs.25,26 Thus, in PPV for PVR RRDs, longer acting gas, such as C3F8 or silicone oil, is often the tamponade of choice. For more challenging cases involving giant retinal tears complicated by PVR, heavy liquid (perfluoro-octane, PFO), can be used as a safe, alternative temporary vitreous substitute to assist in vitrectomy surgery.27 However, it is known to cause high retinal (photoreceptor) toxicity if not removed after an extended period of time.24
PPV has been very successful in treating RRD and is often the primary treatment of choice. Anatomical success rates have been reported of up to 96% for PPV, with low re-detachment rates of 6% reported.14,18 Cataract formation has been shown to be higher in phakic patients undergoing PPV compared to SB and PnR, affecting the final visual outcome. The PIVOT trial found VA had improved by one and 17 Snellen lines following PPV for macula on and macula off RRD, respectively, at one year follow up.14 The SPR study found better VA in phakic patients undergoing SB compared with PPV but found similar improvements in VA in pseudophakic patients undergoing SB and PPV.18
Combined Pars Plana Vitrectomy and Scleral Buckling
PPV and scleral buckling can be combined for more complex RRD cases, or in cases with primarily inferior tear and detachment where gas tamponade is less effective. A recent study from the Primary Retinal Detachment Outcomes (PRO) Study Group found greater single-surgery success with combined PPV/SB for inferior retinal breaks than PPV alone (87.4% vs. 76.8%), particularly in phakic eyes (85.2% vs. 68.6%).28 This was similarly noted for both phakic and pseudophakic patients with moderately complex RRDs, where combined PPV/SB was found to have superior single surgery anatomical success rates compared to PPV alone.20,29
Recent Findings By Muni and Colleagues
The main outcome measure of retinal detachment surgery has conventionally focussed on achieving single-surgery anatomical success. However, many patients are left with post-operative visual deficits in visual function. In a recent multicentre study of 614 patients, up to 75% of patients had metamorphopsia and 32% with aniseikonia after three months following RRD repair,30 similar to earlier studies.31,32 The more recent concept of improving retinal integrity furthers our treatment goals of not only achieving high rates of anatomical success but also optimising functional outcomes. Using modern multimodal imaging techniques, Muni and colleagues have detailed several findings on anatomic and functional outcomes of RRD and have improved our understanding on the pathophysiological changes in RRD.
Quality of Vision
As mentioned earlier, the PIVOT trial demonstrated that patients who underwent PnR had superior VA outcomes, compared to those who underwent PPV, up to 12 months post-operatively.14 Additionally, they demonstrated faster recover of VA at three months post-operatively.14 The study also found higher frequency and severity of vertical metamorphopsia with PPV than PnR.
Post-hoc analysis of the study data found vision-related functioning during the first six months was superior with PnR compared to PPV. These findings demonstrated that successful PnR resulted in superior functional outcomes over PPV and highlights the importance of assessing functional outcome measures such as VA, aniseikonia, metamorphopsia, and vision-related quality of life in RRD.
“The aims of RRD management are to achieve retinal reattachment, seal all breaks, and optimise visual outcomes”
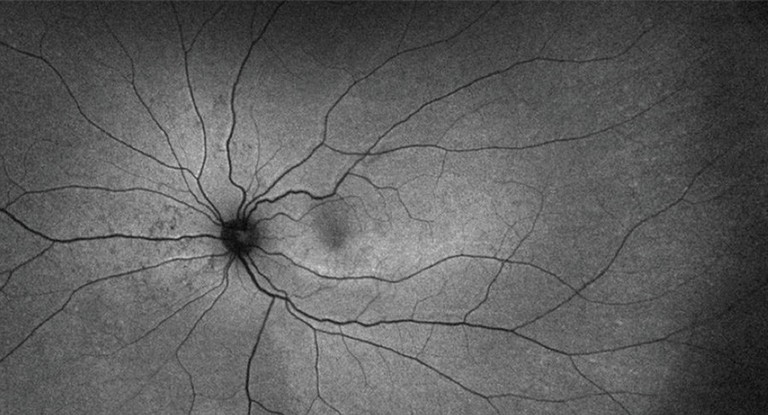
Figure 7. Retinal displacement after RRD repair with PPV demonstrating inferior displacement with retinal imprinting of vessels.33
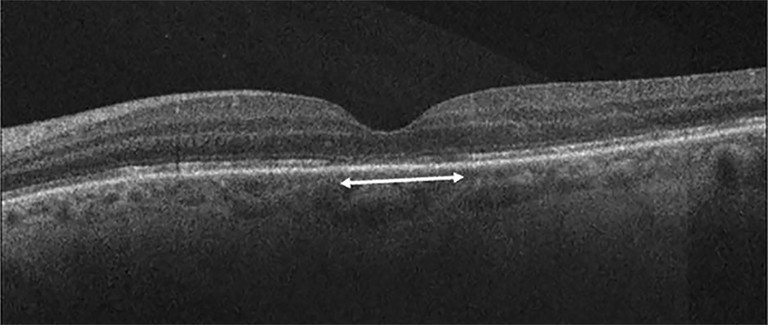
Figure 8. Discontinuity of outer retinal bands (external limiting membrane, ellipsoid zone, interdigitation zone) after macula off RRD repair.30

Figure 9. Morphological stages of RRD on OCT. A) Stage 1, B) Stage 2, C) Stage 3a, D) Stage 3b, E) Stage 4, F) Stage 5.44

Retinal Displacement
The findings of vertical metamorphopsia from the PIVOT trial led to interest in assessing retinal displacement between surgical methods of RRD repair. Two further studies comparing PnR and PPV found significantly less retinal displacement occurring with PnR. One study found the incidence of retinal displacement being 7% with PnR compared to 44% with PPV,33 whereas the multicentre ALIGN study found higher rates (35% in PnR and 60% in with PPV) and retinal displacement appeared to be more severe within those who had PPV.34
“The presence of ORCs is highly specific for RRD”
The ALIGN study also compared outcomes between SB and PPV-SB and found retinal displacement was more prevalent in the PPVSB group (39%) compared to SB (17%).35 This suggests techniques used in PPV may contribute to retinal displacement. There was also a trend toward increased retinal displacement in eyes that underwent SB with external drainage, indicating the act of rapid SRF drainage may promote retinal stretch and subsequent retinal displacement. A metaanalysis encompassing 21 studies with a total of 1,258 eyes found retinal displacement can be found in 35% of all cases of RRD repaired by either SB, PnR, and PPV.36 However, supporting the theory of rapid SRF drainage promoting retinal displacement; SB without tamponade had the lowest rate of retinal displacement, followed by PnR then PPV. Silicone oil tamponade with PPV (compared to gas) and immediate face-down positioning were also found to have reduced incidence of retinal displacement.
Outer Retinal Integrity
The ELLIPSOID study retrospectively analysed 300 patients with macula off RRD who underwent PPV and compared VA outcomes, complication rates, and outer retinal integrity among three SRF drainage techniques.37 The drainage techniques compared were: drainage via peripheral retinal breaks (PRB), posterior retinotomy (PRB), and with PFCL assistance. At the 24-month follow-up in the ELLPSOID-2study,38 drainage through PRB had significantly better visual outcomes compared to those drained by PnR or PFCL assistance. PFCL-assisted drainage had the poorest BCVA outcomes, with the highest incidence of cystoid macular oedema (45.9%), while drainage via PnR was found to have a significantly higher incidence of epiretinal membrane (ERM) formation (93%).
Regarding outer retinal integrity, there was an association between drainage technique and discontinuity of the external limiting membrane (PRB 26%, PnR 24%, PFCL 44%, p=0.001), ellipsoid zone (PRB 29%, PnR 31%, PFCL 49%, p < 0.001), and interdigitation zone (PRB 43%, PnR 39%, PFCL 56%, p=0.004) at 12 months. The PFCL group continued to have a higher rate of interdigitation zone (IDZ) discontinuity (46%) compared to PRB 34% and PnR (27%) at 24 months.
This study demonstrated that the technique of SRF drainage significantly impacts the long-term visual acuity and photoreceptor integrity with drainage through PnR associated with better visual outcomes and fewer complications compared to posterior retinotomy and PFCL-assisted drainage.
Another study similarly followed patients who underwent macula off RRD repair and longitudinally assessed the post-operative ellipsoid zone recovery using en face OCT imaging.39 The study found a significant reduction in the hyporeflective areas of ellipsoid zone (EZ) disruption, indicating a gradual recovery of this critical retinal layer following RRD repair. There was also a correlation of the decrease in EZ hyporeflective area and improvement in visual acuity. This confirms our understanding that as the EZ recovers, VA may improve several months after macula off RRD repair, and that patients with longer duration of macula off RRD before surgery exhibited delayed EZ recovery, emphasising the importance of timely surgical intervention.
Outer Retinal Corrugations
Perhaps one of the most recent distinguished findings on RRD by Muni and colleagues is one that centred on studying outer retinal corrugations (ORCs) using swept source ultra-widefield OCT. ORCs are wave-like folds found in the detached outer retina of RRD. The presence of ORCs is highly specific for RRD. They are essentially absent in exudative forms of retinal detachment.40 This is thought to be due to the difference in osmolarity and/or biochemistry of the subretinal fluid content, resulting in the difference of lateral expansion of the outer retina.
The pathophysiology of ORCs involves the loss of homeostatic regulatory control of the subretinal space and outer retina by the RPE. The dysregulation of the SRF environment by the RPE occurs due to the rapid and continuous influx of a large volume of liquified vitreous into the subretinal space. The formation of ORCs is a compensatory response to progressive outer retinal hydration and lateral expansion in relation to the fixed inner retina.41,42 Findings from a cohort of 66 patients indicate that ORCs occur after approximately two days from the onset of symptoms, suggesting that the RPE-photoreceptor dysregulation results in reversible changes to the mechanical properties of the outer retina until they become overwhelmed after two days.41
On the contrary, in the scenario where the RPE is able to slowly regulate the SRF (once the break is sealed), there is a gradual normalisation in the modulus of elasticity of the outer retina, with a reduction in hydration/lateral outer retinal expansion. This is demonstrated by the absence of ORCs in ‘regulated’ forms of RRD (e.g. slow chronic RRDs in young highly myopic eyes with atrophic holes) compared to the significantly greater incidence of ORCs in ‘dysregulated’ forms of RRD (acute progressive RRDs with rapid uncontrolled SRF accumulation).4 This may also explain the finding of the gradual resolution of ORCs before retinal reattachment when the retina is allowed to naturally reattach by the RPE pump.43
From assessing outer retinal morphological changes with successive swept source OCT scans,5 sequential distinct morphologic stages can be seen with progressive uncontrolled SRF accumulation in dysregulated RRD (Figure 9).
Stage 1: Separation of the neurosensory retina from the RPE without changes in the photoreceptor layer.
Stage 2: Thickening of the photoreceptor inner and outer segments.
Stage 3a: Development of low amplitude ORCs.
Stage 3b: Development of high amplitude ORCs.
Stage 4: Loss of ORC definition with increased thickening of the photoreceptor layer.
Stage 5: Moth-eaten or complete loss of photoreceptor inner and outer segments.
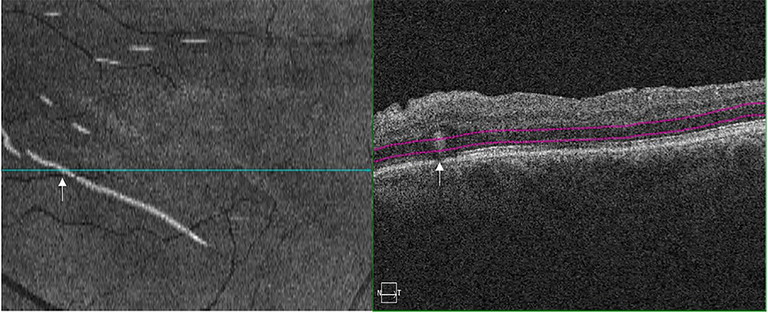
Figure 10. Outer retinal folds, on en face OCT (left image) and B scan OCT (right image).50
“the PIVOT trial demonstrated that patients who underwent PnR had superior VA outcomes, compared to those who underwent PPV, up to 12 months postoperatively”
The morphological stages are useful in prognosticating RRDs as advanced morphological stages are correlated with longer periods of foveal involvement, poorer visual outcomes, and greater disruption of foveal photoreceptor integrity. There was no significant difference in post-operative VA outcomes between stages 1, 2, and 3a; and surgical interventions at these early stages result in better retinal recovery compared to stage 3b or higher. This suggests stage 3b may represent a critical point where substantial structural irreversible photoreceptor changes occur and lead to worse visual outcomes.45 However, in stages 1, 2, and 3a, where the fovea-off duration is within two days, most abnormalities are largely reversible. This gives microstructural evidence that timely intervention is important to maximise visual outcomes in patients with macula off RRD, and supports several other demonstrated improved outcomes with earlier repair.46-49
Outer Retinal Folds
Outer retinal folds (ORFs) are being increasingly recognised as another important outcome of integrity after RRD repair. They are not to be confused with full-thickness retinal folds but are seen as small outer retina foldings on OCT and as hyperreflective or hypo-reflective curvilinear lesions on en face OCT scans.50 These ORFs have demonstrated to be associated with worse post-operative VA outcomes.51
The occurrence and resolution of ORFs were prospectively assessed after RRD repair and were found to occur at the site of preoperative ORCs. During an in vivo imaging study using real-time swept source-OCT following retinal reattachment in eyes undergoing PnR, the process of retinal reattachment was classified into five stages:43
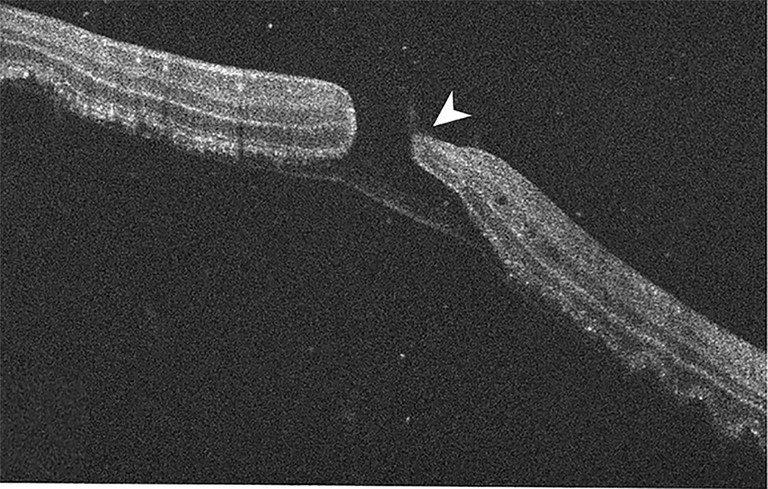
Figure 11. BALAD lamellar hole in macula off RRD with residual posterior hyper-reflective band.52
Stage 1: Redistribution of fluid and approach of the neurosensory retina.
Stage 2: Reduction in cystoid macular oedema and improvement in ORCs, which tend to substantially improve as the retina approaches the RPE.
Stage 3: Contact of the retina with the RPE.
Stage 4: Complete deturgescence of photoreceptors.
Stage 5: Recovery of the PnR integrity in specific substages:
5a: Recovery of the ELM integrity, 5b: Recovery of the EZ integrity,
5c: Recovery of the interdigitation zone and foveal bulge integrity.
The study observed that ORCs resolve in stage 2 before the retina becomes in contact with the RPE (stage 3), and in the event where there is insufficient time for ORCs to resolve before making contact with the RPE, the persistent ORCs develop ORFs.43
ORFs have been found to be more common with PPV than with PnR.51 This may be explained by the more rapid drainage of SRF and retinal reattachment with PPV, expediting the contact of the retina and RPE (stage 3) prior to improvement or resolution of the ORCs (stage 2).
Secondary Macular Hole Formation
Secondary macular hole (MH) formation in the presence of RRD may occur in up to 1–4% of cases with the pathophysiological process not well understood. Highresolution OCT imaging has now identified the development of bacillary layer detachments (BALAD), an OCT sign representing intra-photoreceptor splitting at the inner segment myoid, playing a crucial role in the pathophysiology of secondary MH formation.52
A large study of 360 macula off RRD eyes detailed BALAD-related abnormalities in 22.5% of eyes with secondary MHs in 8.1%, of which 6.4% were BALAD-lamellar holes and 1.7% were full-thickness macular holes (FTMH). As such, the large majority of RRD-associated MHs were BALAD-lamellar holes with an intact thin posterior band of photoreceptors and interphotoreceptor matrix remnant. The study indicates the pathophysiology of secondary MH formation is due to the development of BALAD, then progression to BALAD-lamellar hole and FTMH. With histological evidence to support, the process is thought to initiate due to the progressive hydration and lateral expansion in the context of an acute dysregulated RRD and the formation of ORCs as described above. However, the rigid scaffold of the Muller cell cone prevents the fovea from corrugating and instead, traction within the central bouquet leads to BALAD. Further cystic changes disrupt the anterior cavity wall (outer nuclear layer) of the central bouquet to form a BALAD-lamellar hole, with subsequent disruption of the thin outer PR/interphotoreceptor matrix remnants resulting in a FTMH.
CONCLUSION
There has been significant improvement over the past 100 years in the diagnosis and management of RRDs, with anatomical (reattachment) success rates nearing 96%. The recent works by Dr Muni and colleagues have used modern multi-modal imaging to provide a substantial amount of information, improving our understanding of RRD and reshaping how we manage RRD. It has helped initiate a paradigm shift towards improving the functional outcomes of our patients.
To earn your CPD hours from this article visit: mieducation.com/rhegmatogenous-retinaldetachment-updating-our-management-approach.
References available at mieducation.com.

Dr Tuan Tran BSc MBBS MMed (OphthSc) FRANZCO DRCPSC (Retina) is a medical retinal specialist and vitreoretinal surgeon who runs the vitreoretinal service at the Sunshine Coast University Hospital , Queensland.

Dr Jessica Xiong BMed/MD MMed (OphthSci) is a medical retinal specialist and vitreoretinal fellow of the University of British Columbia, Vancouver.

Dr Sebastian M. Waldstein is a vitreoretinal surgeon and retina specialist with a particular interest in AMD and artificial intelligence. He is Head of Ophthalmology at Mistelbach-Gänserndorf Hospital, and Visiting Professor at the Medical University of Vienna, Austria.

Professor Adrian Fung BSc (Med) MBBS (Hons) MMED (Ophthal Sci) MMED (Clin Epi) with merit, FRANZCO is Head of the Westmead Hospital Vitreoretinal Unit and a specialist in vitreoretinal surgery, medical retina diseases and posterior segment tumours of the eye. He is a Clinical Professor at Macquarie University Hospital and Clinical Associate Professor at the University of Sydney.

Dr Rajeev Muni MD MSc FRCSC FASRS is a staff vitreoretinal surgeon at St Michael’s Hospital, The Hospital for Sick Children and the Kensington Eye Institute, and is the Vice-Chair of Clinical Research in the Department of Ophthalmology and Vision Sciences, University of Toronto.

Dr Isabela Martins Melo MD is a senior fellow in vitreoretinal surgery at St Michael’s Hospital, Unity Health Toronto, Canada.