mieducation

Contact Lens Technologies of the Future: A Review from BCLA CLEAR
In the ninth in a series of articles summarising the key findings of the BCLA CLEAR publication, a major review of the published evidence relating to all aspects of contact lens practice, Adjunct Associate Professor Alex Hui offers an overview of the section relating to contact lens technologies of the future.
WRITER Adjunct Associate Professor Alex Hui
LEARNING OBJECTIVES
On completion of this CPD activity, participants should be able to:
1. Be aware of the potential for contact lenses to diagnose and screen for systemic and ocular diseases,
2. Realise the potential for contact lenses to deliver drugs to the ocular surface,
3. Understand the potential of contact lenses to enhance vision for sport, low vision etc., and
4. Be aware of research being undertaken to enhance contact lens safety.
Soft contact lenses, from their inception, were envisioned to have applications beyond the correction of refractive error. This is evident today, with contact lenses on the market already having indications, such as a bandage or to slow the progression of myopia, that are beyond vision correction.
In the BCLA CLEAR – Contact Lens Technologies of the Futurepublication, experts from around the globe were tapped to provide a snapshot on the future applications of contact lenses and contact lens technology, the research underpinning this technology, and their development status. The article is neatly divided into nine broad topic areas, indicating the breadth of contact lens fields being investigated. Here, I will provide brief overviews of each of these nine areas and the reader is encouraged to read the full article for further details.1
DIAGNOSIS AND SCREENING OF SYSTEMIC DISEASE
When used, contact lenses are in constant contact with one of the body’s fluids, namely the tear film. Naturally, as interest in investigating the tear film for biomarkers of disease has increased, so has the interest in using contact lenses to serve as a sensor for these markers. For example, potential tear biomarkers for Alzheimer’s disease, cystic fibrosis, multiple sclerosis, and thyroid disease have been identified within the literature. 1There have also been various sensors or indicators built into lens materials, from colorimetric or fluorescent means of detection to electrochemical signal generation, which need to be read with an external reader, including potentially, a smartphone.1
One application that has generated significant interest is the use of contact lenses for the detection of glucose levels within the tears as a correlate to blood glucose levels. Numerous research papers have been published detailing the design and detection of glucose in tears using a contact lens platform.1 However, the underlying physiology of the tear film has proven to be a great challenge, as the tear glucose levels relative to blood levels have been found to experience a time delay. 1
Cancer detection with contact lenses has focussed on a range of proteins that are known to be upregulated in certain cancers and their presence and levels within the tear film. In this application, a contact lens detection platform is envisioned to either detect these markers within the tear film or be used to capture these accumulated markers within the lenses during wear, with the data processed after lens removal.1
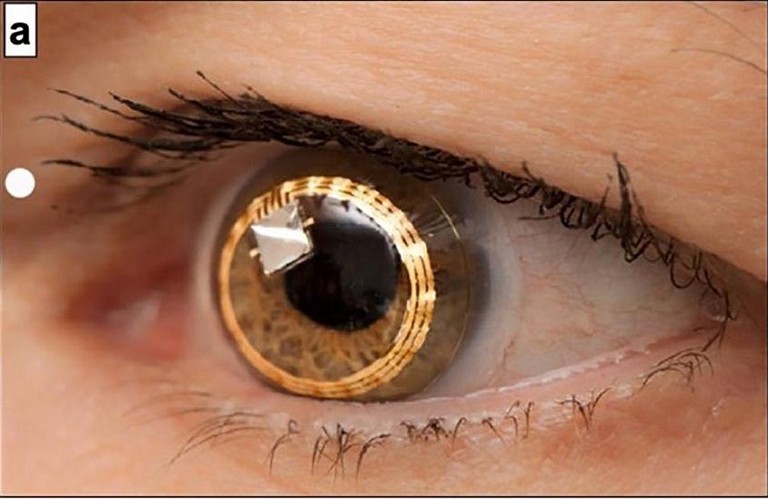
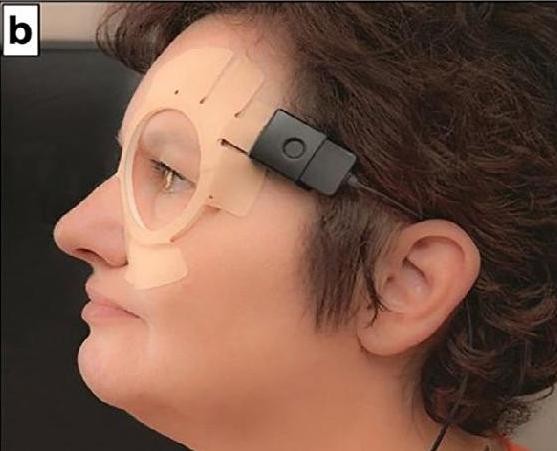
Figure 1. (A) Appearance of Sensimed Triggerfish device on the eye. (B) Antennae receiver placed on the skin surrounding the eye. Reproduced with permission.
DIAGNOSIS AND MANAGEMENT OF OCULAR DISEASE
The Sensimed Triggerfish contact lens sensor device is probably the most wellknown contact lens sensor, as it has been commercialised and approved for use in the European Union and the United States.
The device is designed around measuring changes in the corneal curvature in response to changes in intraocular pressure (IOP) detected via a strain gauge. As it does not directly measure IOP, the device has been marketed as an aid to indicate when the IOP should be best measured during diurnal variation in pressure. This enables the maximum pressure experienced by the eye to be captured. The device measures for 30 seconds every five minutes, leading to 288 measurements over the course of 24 hours. Data is transmitted wirelessly to an antennae receiver, mounted on the eyelids and orbit around the eye. It is then passed to a recording device worn around the neck, allowing patients to use the device during daily living (Figure 1).1
Future iterations and generations of this device, and others, seek to further miniaturise data capture and powering requirements, so that they are less intrusive. The lens design is also being refined – in its current form, the contact lens is quite thick and made of a silicone elastomer material, which may impact comfort.
Other developments have included sensors to measure tear osmolarity for dry eye management, inflammatory cytokines to measure inflammation on the ocular surface, and applications to sense blink rate, ocular temperature, and ocular vasculature responses to lens wear.1
TREATMENT AND MANAGEMENT OF OCULAR CONDITIONS
Medical uses of contact lenses are varied. A detailed discussion of these applications is published in BCLA CLEAR – Medical Uses of Contact Lenses.2 (For a summary, see the August 2023 edition of mivision.)
However, here it is useful to highlight where the medical use of contact lenses has progressed in current contemporary practice as well as what is in the pipeline. Beyond the use of contact lenses as a healing bandage, one of the more transformative applications has been the use of contact lenses to aid limbal stem cell deficiency. In small-scale pilot experiments, limbal stem cells could be seeded on to soft lenses and delivered to a damaged ocular surface to restore the critical functioning of epithelial cells. This may increase rates of transplantation success 1, 3, 4 compared with other methods.
“ …potential tear biomarkers for Alzheimer’s disease, cystic fibrosis, multiple sclerosis, and thyroid disease have been identified ”
The use of technology to manage diseases of the iris also illustrates potential future applications for contact lenses. Artificial static irises, painted or printed on to contact lenses, have been available for many years. This practice has been advanced by researchers developing responsive apertures using technology such as miniaturised liquid crystal cells to respond to changing light conditions. These are envisioned to be applied for patients with iris defects or even transillumination defects to provide a more dynamic response to light. Other applications discussed include lacrimal gland stimulation for dry eye, scavenging of matrix metalloproteinases and reactive oxygen species to remove oxidative stress, and aiding in colour vision deficiency using filters.1
DRUG DELIVERY TO THE OCULAR SURFACE
One of the largest sections of the BCLA CLEAR article detailed the advantages conferred with drug delivery to the ocular surface via contact lenses, and the different strategies used by researchers to control and tailor drug release.
An often-repeated anecdote is that the potential for soft contact lenses to deliver drugs was included in the original patent for these devices. The challenge however, has been to successfully identify a set of suitable drugs and diseases to be treated by contact lens drug delivery.
The advantages discussed in BCLA’s CLEAR paper for contact lens drug delivery include increasing the bioavailability of drugs compared with eye drops, where only 5% of the active ingredient may be able to be absorbed effectively to reach its target site of action.
The use of contact lenses is also a potential avenue for more consistent or accurate dosing of drugs, as long as there is consistency in the lens-to-lens variation of the amount of drug loaded and delivered. In contrast, eye drops have the potential for significant variability due to the need for patients to apply the drops correctly; the amount that bottles are squeezed, the aim of the bottle and drop, and the angle administered are all factors leading to drop dosage variability.
The use of a contact lens drug delivery system may also aid in patient compliance and remove the need for preservatives, as the lenses themselves are typically sterilised at the end of the manufacturing process.
There are, however, several challenges to contact lens drug delivery. Fundamentally, the success of such a combination will depend on the appropriate selection of a contact lens material and a drug, as well as a condition to be treated, as different materials may take up different amounts of different drugs and deliver them at different rates. The drug itself will also need to be able to go through numerous steps of contact lens manufacturing and processing, some of which may require high temperatures or light exposure. Lenses also may have different properties such as thickness differences depending on the lens design, because of the refractive power being conferred on to the wearer.
Notwithstanding all of these factors, the device will need to continue to behave as a contact lens, and so correct for refractive error, be optically transparent, and be compatible with the ocular surface.
The regulatory hurdles will also need to be considered. Approval processes may potentially be complicated by the disease being treated, and the ability of the system to demonstrate effectiveness. The envisioned wear and replacement schedule of the lenses, and the safety of the combination will also require consideration.
One of the main focusses of the field of contact lens drug delivery has been the manipulation or tailoring of drug release from these devices, while also ensuring enough of the drug is able to be loaded on to them. This has included the encapsulation of drugs into carriers, including nanoparticles, cyclodextrins, and poly (lactic-co-glycolic) acid (PLGA) films, or increasing their solubility using microemulsions and then having these placed on to the surface of the contact lens or into the material itself. The use of these carriers allows for a potentially greater amount of the drug to be loaded into the lenses, which can then be slowly released. Other methods have looked into modifying the lens material, including using techniques such as molecular imprinting, which create, on a polymer level, specific areas of greater affinity for a drug of interest, manipulation of the ionicity of the lenses through additives to increase ionic interactions with charged drugs, and the use of coatings or barriers. For example, vitamin E has been studied extensively to serve as a biocompatible, clear barrier that slows the passage of drugs from loaded contact lenses.
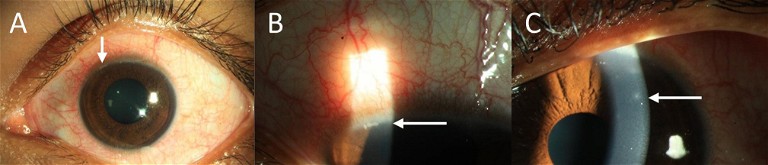
Figure 2. Contact Lens-Induced Acute Red Eye (CLARE) seen with control lens wear; could be reduced with antimicrobial Mel4 coated lenses.6 (A and B) White arrows indicate the infiltration in the limbal and (C) the mid-peripheral cornea. Images courtesy Dr Debarun Dutta, Aston University.
The drugs selected for investigation for delivery from contact lenses have spanned from antimicrobials and anti-inflammatories, to immunomodulators, anti-glaucoma, and anti-allergy agents. Testing has included laboratory studies examining the release of kinetics into static or replenished solutions, as well as in pharmacokinetic studies in animals and animal disease models.
A commercially available contact lens drug delivery device, based on the etafilcon A material, was recently released onto the market in Canada and Japan and has received US Food and Drug Administration (FDA) approval.5 The device releases the anti-allergy drug ketotifen fumarate for the management of ocular allergy and is fit as a daily disposable. Its release to the market shows the commercialisation potential and interest in drug delivery from contact lenses.5
ANTIMICROBIAL CONTACT LENSES
With the goal to improve contact lens safety, by reducing the risk of infection or inflammation associated with microbiological contamination, there is extensive published literature on the incorporation of antimicrobial peptides on to contact lens surfaces.
Melamine, a synthetic antimicrobial peptide derived from salmon sperm and bee venom, and its modified smaller derivative Mel4 have been reported for their impact on infection or contact lens complications after incorporation on to the lens surface.6,7Not only have these been used and tested for their biocompatibility and antimicrobial activity in the laboratory, but Phase II and III clinical trials have demonstrated that the use of such a lens can lead to a lower microbial bioburden on the lens and lower incidences of infiltrative events. 1 Esculentin-1a is another antimicrobial peptide that has been attached to the surface of contact lenses, without impacting critical contact lens parameters, to slow or prevent bacterial adhesion.
THERANOSTICS
Given the potential for contact lenses to both detect and treat disease, a theranostic application for contact lenses has also been proposed. Theranostics, a portmanteau of therapeutics and diagnostics, is a branch of research investigating devices that can both detect a disease state as well as exert a therapeutic effect in response to it. Here, glaucoma management in response to a change in IOP has had the greatest interest due to the commercial availability of the Triggerfish device as well as the work that has been performed on drug delivery. A recent article detailed the design, manufacturing, and evaluation of a combination IOP sensing and glaucoma drug-eluting lens.8 The device was wirelessly powered and used a unique cantilevered capacitor design to detect small changes in IOP, and wirelessly trigger the release of the anti-glaucoma drug brimonidine.8 Another area that has seen interest is dry eye. The hope is that the increase in certain enzymes in the disease, particularly MMP-9, can lead to selective degradation/release of therapeutic agents from the lens’ surface, placing MMP-9 levels at the centre of the severity of the dry eye disease state.
OPTICAL ENHANCEMENTS
Potential optical enhancement applications of contact lenses span from attempting to manage or decrease optical aberrations for enhanced sports performance and low vision, to augmented vision.1
Some of the more interesting developments in accommodative contact lenses are detailed in the patent literature. There are descriptions on how lenses can be designed to detect gaze position then signal that gaze to a controller using an external power supply, to determine the required focus. This is then relayed to a changeable optical system integrated on the lens. Most suggest some sort of electroactive element, such as liquid crystals, which can form rods whose orientation can be controlled by the presence or absence of an electric current, changing the refractive index.1
“The self-contained futuristic lens could have widespread use in the commercial sector, and in daily life”
A relatively newer development in this area is virtual and augmented reality smart contact lens prototypes that are functional with a micro light emitting diode (LED) display and medical grade micro batteries.9 These lenses allow wearers to see images in three dimensions, similar to virtual reality or augmented reality, where information is overlaid on the person’s real environment. The lens has a micro display with 14,000 pixels per inch in a diameter of only 0.5mm.9 The self-contained futuristic lens could have widespread use in the commercial sector, and in daily life.
Enhancement for low vision with contact lenses has centred on having the lenses serve as one part of a Galilean telescope, with the neutralising lens of the system being found on the spectacle plane.1Enhancement for sports has mainly focussed on the use of tints, while augmented vision discussed within the literature has ranged from edge or distance enhancement to entire overlays placed into the visual space to provide real-time information of various interests.1
CONTACT LENS PACKAGING
Developments in contact lens packaging within the industry have primarily focussed on reducing the contamination of lenses when removed and placed on to the eye during lens application. The most prominent example of this technology reaching the market has been the introduction of Smart Touch Technology (Figure 3), where the blister is deliberately designed so that the outer surface of the lens is presented to the user when the packaging is opened. If properly handled and applied from this presentation, the argument is that the inner surface of the lens that is in contact with the eye can remain as contamination free as possible.1
FUTURE STORAGE CASES
Similar to contact lens packaging, the focus of developments for storage cases is on reducing microbial contamination or indicating in some way that a case has reached unacceptable levels of contamination while in use.1
Silver or selenium, incorporated into the lens cases, has primarily been used to reduce storage case contamination. In vitro, this has demonstrated a significant reduction in the number of bacteria recovered, although reportedly only after 24 hours of incubation, with less activity detected if used for only six or 10 hours.1 Unfortunately, clinical use of the cases did not demonstrate a significant impact on case contamination. More than 70% of cases incorporating silver became contaminated after one month, and there was no significant impact on reducing adverse events. 1 To detect contamination, researchers have described the use of gold nanoparticles, tetrazolium dye, or potentially a peptide-graphene nanosensor, as indicators of unacceptable levels of bacteria or other microbes within lens cases.1
CONCLUSIONS
There have been numerous developments in the world of contact lenses for applications beyond the simple correction of refractive error. From medical applications, such as the detection or treatment of a disease, to enhancing the visual world for everyday use or for special cases such as sports or low vision, we now have examples within the literature of groups that are working to extend the potential of contact lenses into the future.
With the recent commercial release of contact lenses to slow the progression of myopia and for anti-allergy drug delivery, in many ways future applications of contact lenses are already here. It will be fascinating to see what will become available in the next few years.
“ A relatively newer development in this area is virtual and augmented reality smart contact lens prototypes ”

Figure 3. Smart Touch packaging in two-blister designs to avoid the need to touch the inner contact lens surface during removal from the blister. Reproduced with permission. Figure 4. Example of biosensor incorporated into a contact lens case. Blue indicates bacterial contamination using tetrazolium dye. Reproduced with permission.

Figure 4. Example of biosensor incorporated into a contact lens case. Blue indicates bacterial contamination using tetrazolium dye. Reproduced with permission.
To earn your CPD hours from this article visit mieducation.com/contact-lens-technologiesof-the-future.
This article is based on an original article published in the United Kingdom in Optician. The full report and supplementary information can be accessed at: sciencedirect.com/science/article/pii/ S1367048421000217.
The Podcast of BCLA CLEAR – Contact Lens Technologies of the Future can be found by scanning the QR code or visit: podcasts.apple.com/us/podcast/bcla-clear-episode-7contact-lens-technologies-of/ id1524673927?i= 1000551281735.
Original paper: Jones, L., Hui, A., Phan, C-M., Read, M.L., Azar, D., Buch, J., Ciolino, J.B., Naroo, S.A., Pall, B., Romond, K., Sankaridurg, P., Schnider, C.M., Terry, L., Willcox, M., CLEAR -Contact lens technologies of the future. Contact Lens and Anterior Eye 2021;44:398–430.

Acknowledgements to the co-authors of the original BCLA CLEAR – Contact Lens Technologies of the Future publication, including Lyndon Jones, Chau-Minh Phan, Michael Read, Dimitri Azar, John Buch, Joseph Ciolino, Shehzad Naroo, Brian Pall, Kathleen Romond, Padmaja Sankaridurg, Christina Schnider, Louise Terry, and Mark Willcox.
The editors of this series are Neil Retallic and Dr Debarun Dutta.
References
1. Jones, L., Hui, A., Phan, C-M., et al., CLEAR -Contact lens technologies of the future. Contact Lens and Anterior Eye 2021;44(2):398–430. DOI: doi.org/10.1016/j. clae.2021.02.007.
2. Jacobs, D.S., Carrasquillo, K.G., Cottrell, P.D., et al., CLEAR – Medical use of contact lenses. Contact Lens and Anterior Eye 2021;44(2):289–329. DOI: doi.org/10.1016/j. clae.2021.02.002.
3. Di Girolamo, N., Bosch, M., Zamora, K., et al., A contact lens-based technique for expansion and transplantation of autologous epithelial progenitors for ocular surface reconstruction. Transplantation 2009;87(10):1571–8. DOI: doi.org/10.1097/TP.0b013e3181a4bbf2.
4. Di Girolamo, N., Chui, J., Wakefield, D., Coroneo, M.T., Cultured human ocular surface epithelium on therapeutic contact lenses. Br J Ophthalmol 2007;91(4):459–64. DOI: doi.org/10.1136/bjo.2006.103895.
5. Pall, B., Gomes, P., Yi, F., Torkildsen, G., Management of ocular allergy itch with an antihistamine-releasing contact lens. Cornea 2019;38(6):713–7. DOI: doi.org/10.1097/ ico.0000000000001911.
6. Dutta, D., Vijay, A.K., Kumar, N., Willcox, M.D.P., Melimine-coated antimicrobial contact lenses reduce microbial keratitis in an animal model. Invest Ophthalmol Vis Sci 2016;57(13):5616–24. DOI: doi.org/10.1167/ iovs.16–19882.
7. Dutta, D., Kamphuis, B., Ozcelik, B., et al., Development of silicone hydrogel antimicrobial contact lenses with mel4 peptide coating. Optom Vis Sci 2018;95(10):937–46. DOI: doi.org/10.1097/OPX.0000000000001282.
8. Yang, C., Wu, Q., Liu, J., et al., Intelligent wireless theranostic contact lens for electrical sensing and regulation of intraocular pressure. Nature Communications 2022;13(1):2556. DOI: doi.org/10.1038/s41467-02229860-x.
9. Whitwam, R., Mojo Vision Details Its First Smart Contact Lens; 2022. Available at: extremetech.com/extreme/337691-mojo-vision-details-its-first-smart-contactlens [Accessed 20 May 2023].

Alex Hui OD PhD FAAO is an Adjunct Associate Professor at the School of Optometry and Vision Science at the University of New South Wales Sydney and Head of Biosciences at the Centre for Ocular Research and Education, University of Waterloo.