mieducation
Artificial Intelligence: Reshaping AMD Management
The integration of artificial intelligence (AI) into age-related macular degeneration management heralds a new era of personalised care and precision medicine. With its ability to predict disease progression, identify biomarkers, and assist in treatment decision-making, AI offers a powerful tool to enhance visual outcomes for AMD patients.
WRITER Dr Yvette Wang
LEARNING OBJECTIVES
On completion of this CPD activity, participants should be able to:
1. Understand the role artificial intelligence can play in detection and management of AMD,
2. Be aware of the accuracy that can be achieved when using AI to screen for AMD, and
3. Realise the logistical and financial benefits of AI screening.
The eye unveils subtle signs of ageing, with age serving as the foremost risk factor for age-related macular degeneration (AMD). AMD is a disease that affects the macular region of the retina, causing progressive loss of central vision. Nearly all instances of late AMD occur in individuals aged over 60. The prevalence of AMD surges exponentially with age, with the risk escalating after the age of 50. Late AMD prevalence is estimated at 1.4% at age 70, escalating to 5.6% at age 80, and a staggering 20% at age 90.1 Due to the high incidence and risk, improving the efficiency of AMD screening and diagnosis is an imperative.
AMD includes early, intermediate, and late stages (Figure 1). Early AMD is frequently asymptomatic, though some patients may experience difficulty with central vision, impacting activities like reading and driving. Intermediate AMD is characterised by drusen in the macular region or pigmentary abnormalites, usually larger than early stage AMD. Patients may complain about deteriorated central vision, but progression is slow. Late AMD, affecting central vision, progresses rapidly (over weeks or months) in the wet (neovascular) form (nAMD). However, it progresses more gradually (over years or decades) in the dry (atrophic) form. Late stage AMD is accompanied by vision loss, making treatment opportunities in advanced stages time sensitive.
Traditionally, AMD has been diagnosed on the basis of clinical examinations or assessments of colour fundus photographs. However, in recent decades technological advances like spectral-domain optical coherence tomography (OCT) and fundus autofluorescence (FAF) imaging have revolutionised lesion detection. The management of neovascular AMD has also seen significant strides over the past couple of decades, largely due to the recognition of vascular endothelial growth factor’s (VEGF) role in its development. Administering VEGF inhibitors, like ranibizumab, aflibercept, and bevacizumab directly into the eye, has proven highly effective in reducing leakage from abnormal blood vessels and improving retinal health, thereby offering tangible benefits in terms of vision improvement.
The Royal Australian and New Zealand College of Ophthalmology (RANZCO) advises individuals with early or intermediate AMD to undergo screening at least once every year.2 The core principle underpinning AMD screening is the belief that early detection significantly improves treatment outcomes.3 Until now the main strategy has been to detect nAMD earlier, as there is treatment for it. This allows for more frequent treatment of smaller and potentially fewer active lesions, leading to overall better results. However, despite this understanding, challenges persist in effectively managing AMD. Advances in telescreening for AMD lag behind diabetic retinopathy screening, primarily due to the difficulty in detecting and grading early signs such as patchy atrophy and small drusen, which are less conspicuous compared to the more apparent microaneurysms and haemorrhages.4 Additionally, current comprehension of the factors driving late AMD progression and methods for predicting its advancement remain limited. Furthermore, strategies for measuring lesion size are both slow and resource intensive, contributing to the ongoing challenges faced in AMD management.
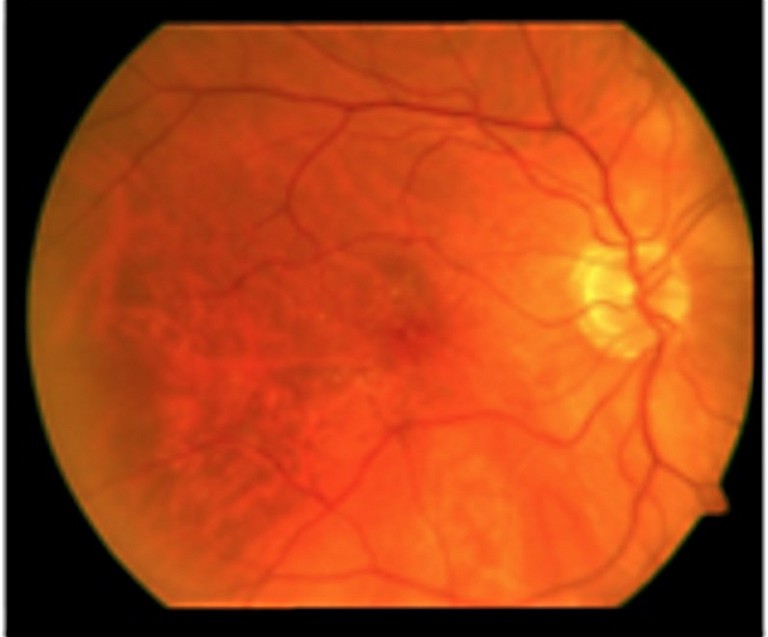
Figure 1. Different stages of AMD. A) Early stage with small drusen.
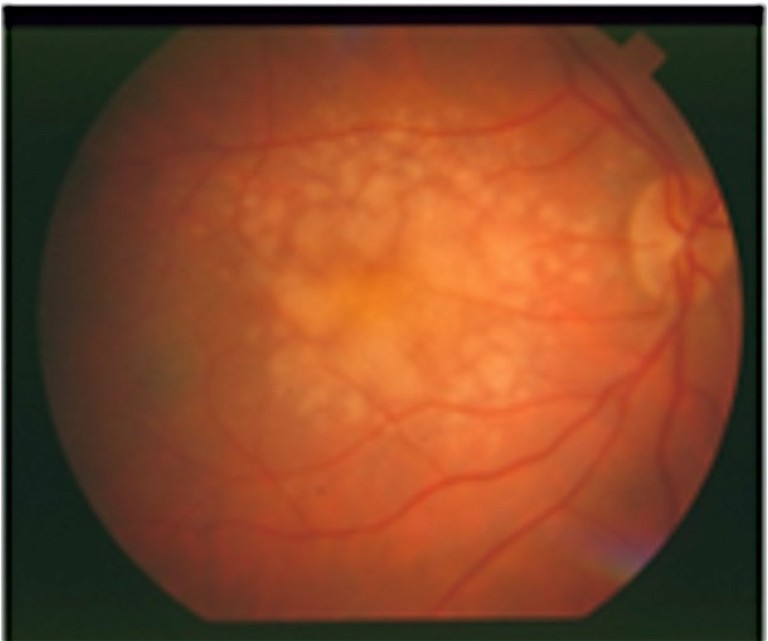
B) Intermediate stage with large soft drusen.
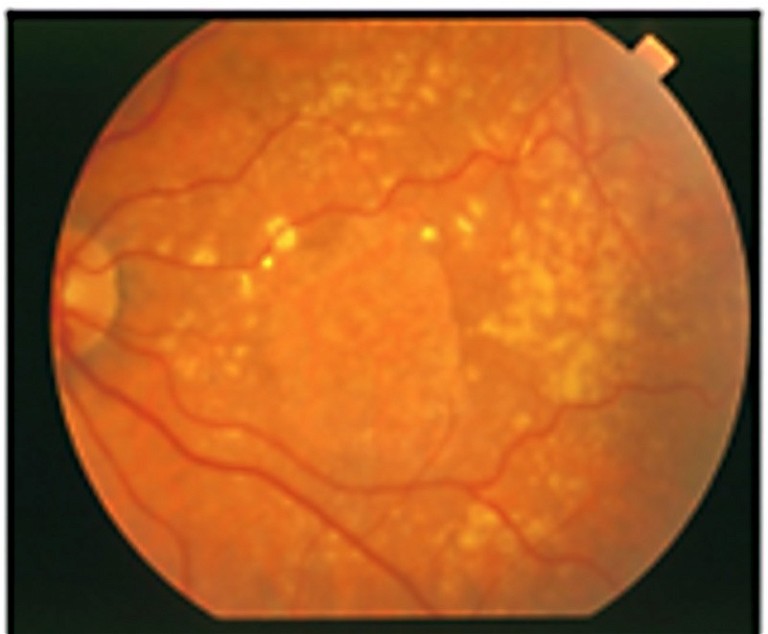
C) Late dry stage with geographic atrophy.
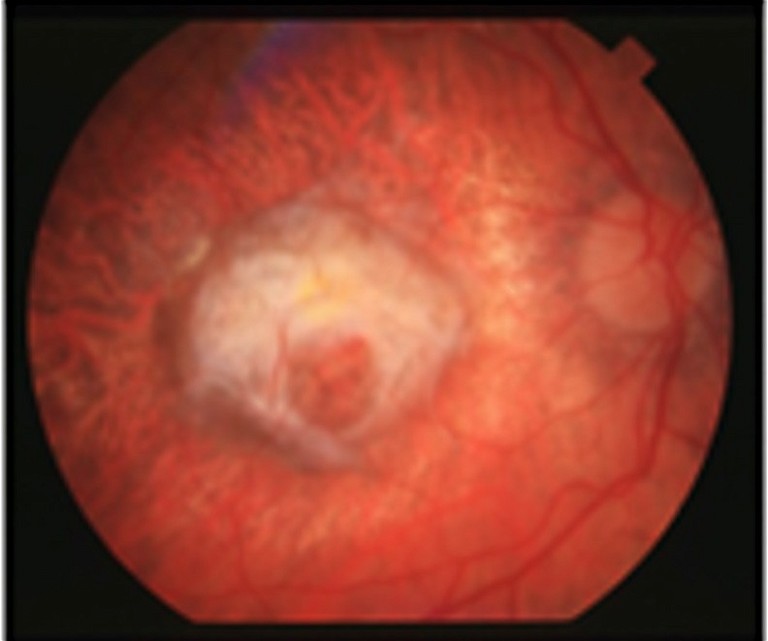
D) Late wet stage with neovascularisation.
EMERGING ROLE OF AI IN AMD MANAGEMENT
Healthcare systems worldwide face formidable challenges in meeting the needs of the estimated 200 million individuals affected by AMD.5 The escalating demand for AMD screening and patient monitoring strains healthcare resources, underscoring the potential of AI to offer significant support in AMD management.
Empowered by vast datasets, robust computational capabilities, and expansive networks, AI holds the promise of analysing retinal fundus images to deliver invaluable insights for diagnosing and tracking eye conditions. From identifying biomarkers and aiding in diagnosis, to facilitating decision making and treatment, and finally to monitoring disease progression, AI has the potential to revolutionise every aspect of AMD management (Figure 2).
AI algorithms have been refined to detect and classify various eye abnormalities with accuracy akin to that of retinal experts, showcasing promising potential. By scrutinising extensive data from genetic studies, lifestyle factors, and imaging modalities, AI can predict AMD risk and progression. Moreover, AI aids in the early detection of AMD by discerning subtle retinal changes imperceptible to human eyes.
AI also contributes to tailoring treatment plans by integrating individual genetic profiles, lifestyle considerations, and disease characteristics.
Timely diagnosis and treatment are imperative for preserving functional vision in AMD patients, and AI-based tools offer a promising avenue for enhancing disease management.
BIOMARKER IDENTIFICATION
Identifying early biomarkers indicative of progression risk is crucial for assessing which patients are most likely to transition from the early stages to vision-threatening late-stage AMD. While several established structural biomarkers, such as drusen volume and pigmentary abnormalities, offer valuable insights, ongoing research aims to explore a spectrum of multimodal biomarkers poised to enhance risk assessment further in the future.6
AI emerges as a transformative tool for the automated extraction of these biomarkers. The wealth of information gleaned from OCT analysis of AMD patients poses a challenge for manual assessment of imaging biomarkers, given its subjective nature and impracticality in clinical settings. Thus, there is a pressing need for automatic image analysis to furnish objective and reproducible measurements of quantitative features.
Leveraging AI techniques, such as vascular skeleton extraction, enables computation of metrics like tortuosity, fractal index, thickness, and vessel density in fundus images.7 Moreover, AI facilitates the detection and quantification of drusen, alongside automatic image segmentation, heralding a cost-effective, rapid throughput approach to biomarker extraction.
AI techniques excel at identifying the most important factors from large amounts of data. Once potential biomarkers have been completely identified and agreed upon, AI-based methods could play a pivotal role in pinpointing those that are crucial for guiding targeted therapy in ophthalmology. Machine learning within AI excels in discerning the most significant risk factors amid myriad predictors, especially in the realm of high-dimensional data produced by diverse imaging modalities. Deep learning approaches have demonstrated remarkable efficacy, combining OCT angiography (OCT-A) and structural OCT data to predict AMD biomarkers with up to 90% accuracy.8 Researchers harness AI to quantify retinal morphologic features and leverage spatiotemporal atlases for morphometric analysis, offering a comprehensive understanding of disease progression. Quantitative characteristics of drusen, including number, volume, height, reflectivity, and longitudinal evolution, emerge as promising biomarkers for predicting the risk of transitioning to nAMD.9 This effort contributes to generating additional evidence, expediting screening protocols, and fostering consensus on a standardised set of biomarkers critical for identifying vision-threatening AMD.
LESION DETECTION AND DIAGNOSIS
In clinical practice, various diagnostic tests, like fluorescein angiography (FFA), OCT, OCT-A, fundus photos, FAF, and indocyanine green angiography (ICGA) are valuable tools for detecting AMD. Recent advancements in deep learning-based AI algorithms have significantly enhanced our ability to diagnose AMD accurately using these multi-modal retinal images.10 Some studies have successfully used convolutional neural network models to identify advanced AMD based on spectral domain OCT images.11,12 Moreover, integrating data from multiple sources has shown the potential to enhance the overall diagnostic accuracy of AI models for AMD detection compared to using fundus images alone.13
Additionally, AI techniques can classify the severity of AMD, providing valuable insights for patient management. These algorithms boast high accuracy in identifying key features of AMD, such as different types of drusen (small, medium, large), pigmentary changes in the retinal pigment epithelium (hypo- and hyper-reflective pigmentary changes), reticular pseudodrusen, and late forms of AMD, including both neovascular and geographic atrophic. With its potential to revolutionise AMD diagnosis, these AI algorithmic approaches present sophisticated tools for the efficient identification of AMD and its sight-threatening subtypes, promising significant advancements in timely diagnosis and intervention.
Several research efforts have concentrated on geographic atrophy (GA), a prominent characteristic of dry AMD.14 From these efforts, various AI algorithms have been developed to detect GA lesions, with a primary focus on segmenting these lesions from retinal images.15 This segmentation process offers several advantages, including enhancing traditional manual annotation methods, which are often laborious and time-consuming.
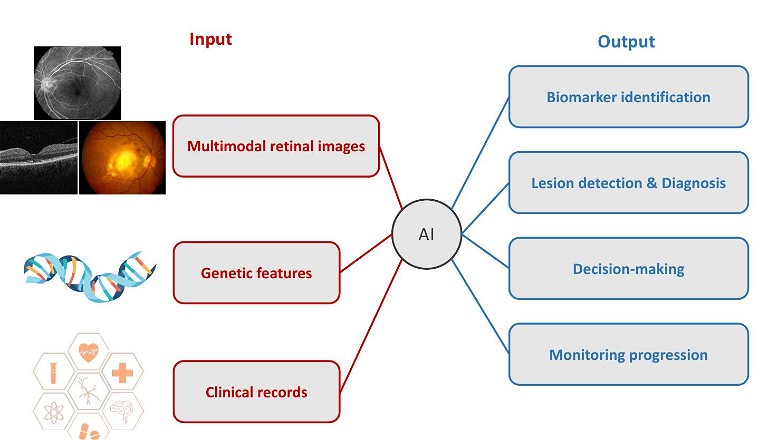
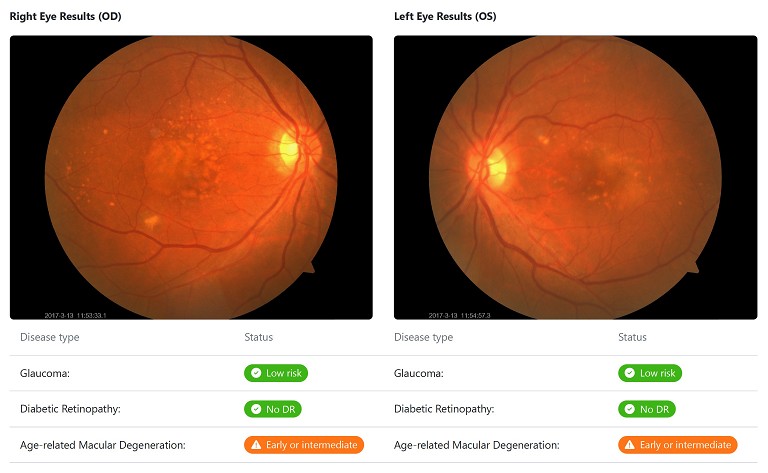
Figure 3. Highlighted regions on the heatmap identify presence of AMD.
An AI algorithm, empowered by Optain, has demonstrated remarkable accuracy in detecting nAMD, which is widely recognised as the most vision-threatening subtype of the disease.16 This algorithm represents a significant leap forward in automated diagnostic technology for ophthalmic applications specifically developed to address the critical need for precise and efficient identification of nAMD. Trained on an extensive dataset encompassing retinal images sourced from diverse clinical settings, this AI algorithm harnesses cutting-edge deep-learning techniques to discern subtle features indicative of nAMD from colour fundus photographs. Leveraging convolutional neural networks and a wealth of training data, the algorithm exhibits remarkable sensitivity and specificity in identifying nAMD, offering a reliable and scalable solution for enhancing AMD screening and diagnostic processes.
DECISION MAKING AND TREATMENT
With the introduction of novel therapeutic options for patients with nAMD, the treatment landscape is poised to become increasingly intricate. However, individual responses to intravitreal anti-VEGF therapy can vary significantly among patients, adding to the complexity of treatment decisions and burden on patients. The need for swift, objective, and quantitative evaluation of disease activity has emerged as a pivotal challenge in treatment response surveillance and decision making.
AI has revolutionised the monitoring of precise treatment outcomes, particularly in the realm of ophthalmology. AI-based OCT segmentation holds promise in enhancing the analysis and interpretation of OCT images, thereby facilitating more informed clinical assessments based on imaging and clinical data.
Recent research endeavours have yielded machine learning models for the segmentation of retinal fluid, a critical pathological feature guiding clinicians’ decisions regarding anti-VEGF therapy administration.17 Through AI-based image analysis, researchers can accurately localise and quantify topographic variations in GA progression, identify morphological and structural properties influencing progression rates, and account for these factors to ensure an accurate evaluation of therapeutic efficacy.18
Studies have demonstrated the capabilities of AI methodologies in predicting visual outcomes and retreatment intervals for a treat-and-extend regimen from a single injection.19 These models, trained and evaluated on data from prospective clinical trials involving treatment-naïve nAMD patients, represent a crucial step towards AI-supported management of active and progressive nAMD.
AI-enabled software has also been employed to identify and monitor early structural changes on OCT imaging following subthreshold nanosecond laser treatment in AMD patients, showcasing the potential for AI to revolutionise treatment monitoring and decision making.20
Furthermore, AI models trained on AI-segmented OCT features and clinical variables have shown promise in predicting future response to anti-VEGF treatment in patients with nAMD.21 Novel algorithms, like HDF Net, designed to predict visual acuity outcomes following anti-VEGF treatment, highlight the potential of deep learning to process a wide range of clinical data and inform personalised therapeutic strategies for typical nAMD.22 This innovative approach represents a significant advancement towards the implementation of real-world personalised treatment strategies in the management of nAMD.
MONITORING PROGRESSION
It is recognised that patients within different AMD sub-phenotypes may have different rates of progression at different stages of the disease. Prediction and timely identification of the risk of progression from early and intermediate stages of AMD to advanced forms, and prompt medical intervention, could revolutionise patient screening and facilitate earlier detection, with the potential to mitigate disease progression and ultimately improve visual outcomes. Therefore, capturing progression information from previous visits may prove invaluable in predicting AMD progression.
AI methods excel at detecting subtle signs of progression, which are often too time-consuming for clinicians to identify in a busy clinical practice. This has led to recent applications in predicting AMD progression based on previous clinical data and retinal examinations.
Fundus images alone can predict progression to late AMD with reasonable accuracy, however notable improvements in accuracy can be achieved by further incorporating retinal images and genotype data to assess AMD severity. A combination of retinal features and genetic factors can help predict how long it will take for AMD to progress beyond a certain point. Researchers have used advanced deep learning techniques to identify detailed features within images, which are then combined with genetic information to understand the condition of patients’ eyes and predict the risk of AMD progression.23,24
Automated OCT image analysis using deep learning techniques is widely used to predict disease progression in AMD. Previous studies have investigated the prediction of conversion from early or intermediate AMD to nAMD using quantitative imaging features extracted from OCT images, such as drusen shape and volume.7 Some studies have focussed on predicting the future progression of GA.25 These longitudinal studies involve predicting disease severity over time, as well as predicting future progression based on previous observations from the same patient.26-28
Generative AI models can generate future fundus images with appropriate pathological features, presenting drusen development trajectory over time.29 AI has also been used to predict vision change and vision-related quality of life along with disease progression.30 There are also efforts to use innovative techniques, such as time-aware long short-term memory to model patients’ temporal visits, accounting for irregular time gaps between appointments.31 These advances highlight the potential of AI-driven approaches to revolutionise the prediction and management of AMD progression.
NAVIGATING CHALLENGES
While AI is still in its early stages of development in the AMD diagnosis and disease monitoring process, its integration into this field brings several challenges that must be addressed for widespread clinical use. However, in the foreseeable future, AI holds the potential to significantly impact fundus applications, screening procedures, telemedicine, and alleviate the workload of physicians.
Currently, AI primarily targets automating the diagnosis of fundus diseases. However, its utility is hindered by the limited selection of diseases included in public or self-built databases, which restricts applicability in clinical settings. Establishing comprehensive databases that encompass diverse and large image sets remains a significant hurdle, crucial for training robust AI models capable of accurately diagnosing AMD and other fundus pathologies. Moreover, while deep learning algorithms predominantly analyse fundus images, clinical data such as fundus photographs and OCT results are indispensable for achieving diagnostic precision. The integration of multimodal data, including fundus photographs, OCT, OCT-A, and other emerging technologies, holds promise for enhancing diagnostic accuracy. However, the current lack of inclusion of newer imaging modalities, like OCT-A in traditional fundus datasets poses a challenge to harnessing its full potential in AMD diagnosis.
Additionally, the validation of AI models primarily relies on research datasets. Caution is needed when applying AI-based screening in larger populations, including different ethnic groups, under different settings and conditions. There is still a great deal of necessary research to be conducted, including the validation of these models using prospective studies to evaluate for accuracy, reproducibility (sensitivity/specificity), and other important factors. This raises concerns about AI’s real-world applicability and generalisation to diverse patient populations and clinical settings.
“ The integration of AI into AMD management heralds a new era of personalised care and precision medicine ”
There are also regulatory hurdles that need to be overcome prior to obtaining approval to use these deep learning models in a clinical setting beyond research purposes. Moreover, the absence of standardised reference guidelines for defining AMD complicates the evaluation and comparison of different AI systems. Ultimately, the effectiveness of AI in AMD management is contingent upon the establishment of high-quality, diverse, and representative databases, underscoring the need for concerted efforts to address these challenges for the successful integration of AI into routine clinical practice.
ENVISIONING TOMORROW
The integration of deep learning into clinical practice warrants clarification, as current AI models often operate as black boxes. While these models may demonstrate safety and accuracy, clinicians, and patients may hesitate to trust AI systems due to its opaque decision-making processes. It has been reported that an explainable deep-learning framework can effectively identify clinically relevant pathological features for diagnosing AMD from retinal images.32 Efforts in explainable AI, such as providing explanations for the model’s diagnosis and visualising regions of the retina that influenced the diagnosis through heat maps, can instil confidence in clinicians regarding AI’s capabilities.
An explainable model can also foster collaboration between artificial and human intelligence. Although deep learning can accurately predict disease progression, it is crucial to understand the clinician’s role in interpreting AI recommendations. While deep learning models provide accurate detections and predictions, clinicians use their expertise to interpret AI results and determine the next course of action, including recommendations.
GENERATIVE AI AND LARGE LANGUAGE MODELS
The emergence of generative artificial intelligence and large language models (LLMs) has opened transformative avenues in medicine. Particularly in ophthalmology, LLMs present unique opportunities to revolutionise digital eye care, streamline clinical workflows, and enhance patient experiences globally.33 While LLMs like GPT show promise in providing precise information about AMD, further improvements are necessary, especially in addressing more technical queries.34 Deep learning can augment physicians’ diagnostic abilities, aiding in the detection of individuals who may benefit from secondary prevention measures. In the era of electronic medical records, deep learning offers insights into real-world treatment outcomes for AMD, with the potential for LLMs to further enrich the AMD management cycle in the future.
FFA and ICGA served as gold standards for AMD diagnosis and severity classification, yet these procedures are invasive. Generative AI offers non-invasive trans-modality alternatives for obtaining FFA and ICGA results. It has been reported that high-resolution ICGA images can be synthesised from retinal fundus images using generative adversarial networks (GANs), with the reliability of this model validated through internal and external validation.35 Integrating translated ICGA images with fundus images, together with OCT imaging, significantly enhances the accuracy of AMD screening. This approach not only demonstrates the feasibility of predicting choroidal abnormalities more accurately from accessible fundus images via GANs, but also introduces a cross-modality approach to enrich data for AMD-related deep learning research.
COST-EFFECTIVENESS EVALUATION
Evaluating the cost-effectiveness of using AI in AMD management is vital from a healthcare system perspective. Traditional ophthalmologic screening for AMD, while effective in reducing blindness, may not be cost-effective.36 However, AI-based screening, especially when combined with screening for multiple eye diseases, offers a potentially cost-effective approach. Research indicates that combined screening for various blindness-causing diseases, including AMD, is likely to be highly cost-effective and align with the World Health Organization’s criteria for population screening.37 Annual AI screening emerges as the most cost-effective strategy, emphasising the importance of assessing cost-effectiveness and developing optimal application strategies before implementing AI in practical AMD management.
Despite existing challenges, such as database limitations and regulatory hurdles, the future of AI in AMD management is bright and promising. As we continue to innovate and overcome obstacles, AI holds the key to revolutionising the way we diagnose, treat, and monitor AMD, ultimately preserving sight and transforming lives.
To earn your CPD hours from this article, visit mieducation.com/artificial-intelligence-reshapingamd-management.
This article was sponsored by Optain.
References
1. García-Layana, A., Cabrera-López, F., and Ruiz-Moreno, J. M., et al., Early and intermediate age-related macular degeneration: update and clinical review. Clin Interv Aging 12, 1579–1587, (2017). DOI: 10.2147/cia.S142685.
2. RANZCO, RANZCO Referral Pathways for AMD Screening and Management by Optometrists, available at: ranzco.edu/wp-content/uploads/2018/11/RANZCO-Referral-pathwayfor-AMD-management.pdf [accessed March 2024].
3. Jain, S., Hamada, S., Membrey, W. L. and Chong, V., Screening for age-related macular degeneration using nonstereo digital fundus photographs. Eye (Lond) 20, 471–475, (2006). DOI: 10.1038/sj.eye.6701916.
4. van Leeuwen, R. Chakravarthy, U., de Jong, P.T.V.M. et al., Grading of age-related maculopathy for epidemiological studies: is digital imaging as good as 35-mm film? Ophthalmology 110, 1540–1544, (2003). DOI: 10.1016/s0161-6420(03)00501-3.
5. Deng, Y., Qiao, L., Huang, L., et al., Age-related macular degeneration: Epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes Dis 9, 62–79, (2022). DOI: 10.1016/j.gendis.2021.02.009.
6. Lad, E. M., Finger, R. P., Guymer, R., Biomarkers for the progression of intermediate age-related macular degeneration. Ophthalmol Ther 12, 2917–2941, (2023). DOI: 10.1007/s40123-023-00807-9.
7. de Sisternes, L., Simon, N., Rubin, D. L., Quantitative SD-OCT imaging biomarkers as indicators of age-related macular degeneration progression. Invest Ophthalmol Vis Sci 55, 7093–7103 (2014). DOI: 10.1167/iovs.14-14918.
8. Saha, S., Nassisi, M., Wang, M., et al., Automated detection and classification of early AMD biomarkers using deep learning. Sci Rep 9, 10990 (2019). DOI: 10.1038/s41598-019-47390-3.
9. Waldstein, S. M., Vogl, W-D., Schmidt-Erfurth, U., et al., Characterization of drusen and hyperreflective foci as biomarkers for disease progression in age-related macular degeneration using artificial intelligence in optical coherence tomography. JAMA Ophthalmol 138, 740–747, (2020). DOI: 10.1001/jamaophthalmol.2020.1376.
10. Dong, L., Yang, Q., Zhang, R. H., Wei, W. B., Artificial intelligence for the detection of age-related macular degeneration in color fundus photographs: A systematic review and meta-analysis. EClinicalMedicine 35, 100875, (2021). DOI: 10.1016/j.eclinm.2021.100875.
11. Mantel, I., Mosinska, A., De Zanet, S., et al., Automated quantification of pathological fluids in neovascular age-related macular degeneration, and its repeatability using deep learning. Transl Vis Sci Technol 10, 17, (2021). DOI: 10.1167/tvst.10.4.17.
12. Venhuizen, F. G., van Ginneken, B., van Asten, F., et al., Automated staging of age-related macular degeneration using optical coherence tomography. Invest Ophthalmol Vis Sci 58, 2318–2328, (2017). DOI:10.1167/iovs.16-20541.
13. Yoo, T. K., Choi, J.Y., Seo, J.G. et al., The possibility of the combination of OCT and fundus images for improving the diagnostic accuracy of deep learning for age-related macular degeneration: a preliminary experiment. Med Biol Eng Comput 57, 677–687, (2019). DOI: 10.1007/s11517-018-1915-z.
14. Arslan, J., Samarasinghe, G., Baird, P.N., et al., Artificial intelligence algorithms for analysis of geographic atrophy: A review and evaluation. Transl Vis Sci Technol 9, 57, (2020). DOI: 10.1167/tvst.9.2.57.
15. Ramsey, D. J., Sunness, J.S., Handa, J.T., et al., Automated image alignment and segmentation to follow progression of geographic atrophy in age-related macular degeneration. Retina 34, 1296–1307, (2014). DOI: 10.1097/ iae.0000000000000069.
16. Keel, S. Zhixi, L. He, M., et al., Development and validation of a deep-learning algorithm for the detection of neovascular age-related macular degeneration from colour fundus photographs. Clin Exp Ophthalmol 47, 1009–1018, (2019) DOI: 10.1111/ceo.13575.
17. Keenan, T. D., Dharssi, S., Chew, E.Y., et al., A deep learning approach for automated detection of geographic atrophy from color fundus photographs. Ophthalmology 126, 1533–1540, (2019). DOI: 10.1016/j. ophtha.2019.06.005.
18. Vogl, W. D., Riedl, S., Schmidt-Erfurth, U., et al., Predicting topographic disease progression and treatment response of pegcetacoplan in geographic atrophy quantified by deep learning. Ophthalmol Retina 7, 4–13, (2023). DOI: 10.1016/j.oret.2022.08.003.
19. Bogunović, H., Mares, V., Reiter, G. S., Schmidt-Erfurth, U., Predicting treat-and-extend outcomes and treatment intervals in neovascular age-related macular degeneration from retinal optical coherence tomography using artificial intelligence. Front Med (Lausanne) 9, 958469, (2022). DOI:10.3389/fmed.2022.958469.
20. Hanna, V., Oakley, J., Russakoff, D., Choudhry, N., Effects of subthreshold nanosecond laser therapy in age-related macular degeneration using artificial intelligence (STAR-AI Study). PLoS One 16, e0250609, (2021). DOI: 10.1371/ journal.pone.0250609.
21. Maunz, A., Barras, L., Sahni, J., et al., Machine learning to predict response to ranibizumab in neovascular agerelated macular degeneration. Ophthalmol Sci 3, 100319, (2023). DOI: 10.1016/j.xops.2023.100319.
22. Yeh, T. C., Luo, A.C., Deng, Y.S., et al., Prediction of treatment outcome in neovascular age-related macular degeneration using a novel convolutional neural network. Sci Rep 12, 5871, (2022). DOI: 10.1038/s41598-022- 09642-7.
23. Yan, Q., Weeks, D.E., Xin, H., et al., Deep-learningbased prediction of late age-related macular degeneration progression. Nat Mach Intell 2, 141–150, (2020). DOI: 10.1038/s42256-020-0154-9.
24. Peng, Y., Keenan, T.D., Chen, Q., et al., Predicting risk of late age-related macular degeneration using deep learning. NPJ Digit Med 3, 111, (2020). DOI: 10.1038/s41746-020-00317-z.
25. Yim, J., Chopra, R., Spitz, T., et al., Predicting conversion to wet age-related macular degeneration using deep learning. Nat Med 26, 892–899, (2020). DOI: 10.1038/s41591-020-0867-7.
26. Niu, S., de Sisternes, L., Leng, T., et al., Fully automated prediction of geographic atrophy growth using quantitative spectral-domain optical coherence tomography biomarkers. Ophthalmology 123, 1737–1750, (2016). DOI: 10.1016/j.ophtha.2016.04.042.
27. Pfau, M., Möller, P.T., Fleckenstein, M., et al., Type 1 choroidal neovascularization is associated with reduced localized progression of atrophy in age-related macular degeneration. Ophthalmol Retina 4, 238–248, (2020). DOI: 10.1016/j.oret.2019.09.016.
28. Schmidt-Erfurth, U., Bogunovic, H., Reiter, G.S., et al., Role of deep learning-quantified hyperreflective foci for the prediction of geographic atrophy progression. Am J Ophthalmol 216, 257–270, (2020). DOI: 10.1016/j. ajo.2020.03.042.
29. Pham, Q. T. M., Ahn, S., Shin, J., Song, S. J., Generating future fundus images for early age-related macular degeneration based on generative adversarial networks. Comput Methods Programs Biomed 216, 106648, (2022). DOI: 10.1016/j.cmpb.2022.106648.
30. Künzel, S. H., Möller, P.T., Pfau, M., et al., Determinants of quality of life in geographic atrophy secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci 61, 63, (2020). DOI: 10.1167/iovs.61.5.63.
31. Yin, C., Moroi, S. E., Zhang, P., Predicting age-related macular degeneration progression with contrastive attention and time-aware LSTM. Kdd 2022, 4402–4412, (2022). DOI: 10.1145/3534678.3539163.
32. Ma, D., Kumar, M., Beg, M.F., et al., Clinical explainable differential diagnosis of polypoidal choroidal vasculopathy and age-related macular degeneration using deep learning. Comput Biol Med 143, 105319, (2022). DOI: 10.1016/j.compbiomed.2022.105319.
33. Betzler, B. K., Chen, H., Wong, T.Y., et al., Large language models and their impact in ophthalmology. Lancet Digit Health 5, e917-e924, (2023). DOI: 10.1016/s2589-7500(23)00201-7.
34. Ferro Desideri, L., Roth, J., Zinkernagel, M., Anguita, R., Application and accuracy of artificial intelligence-derived large language models in patients with age related macular degeneration. Int J Retina Vitreous 9, 71, (2023). DOI:10.1186/s40942-023-00511-7.
35. Chen, R., Zhang, W., Shi, D., et al., Translating color fundus photography to indocyanine green angiography using deep-learning for age-related macular degeneration screening. NPJ Digit Med 7, 34, (2024). DOI: 10.1038/s41746-024-01018-7.
36. Tamura, H., Akune, Y., Hiratsuka, Y., et al., Real-world effectiveness of screening programs for age-related macular degeneration: amended Japanese specific health checkups and augmented screening programs with OCT or AI. Jpn J Ophthalmol 66, 19–32, (2022). DOI: 10.1007/s10384-021-00890-0.
37. Liu, H., Li, R., Wang, N., et al., Economic evaluation of combined population-based screening for multiple blindness-causing eye diseases in China: a cost-effectiveness analysis. Lancet Glob Health 11, e456-e465, (2023). DOI: 10.1016/s2214-109x(22)00554-x.

Dr Yvette Wang is an ophthalmologist and the current senior clinical advisor at Optain Health. She holds a PhD from the Zhongshan Ophthalmic Center, China’s leading institution for ophthalmology.
Dr Wang's primary research focus revolves around the integration of artificial intelligence in ophthalmology and the practical implementation of AI-driven medical devices. Her scholarly endeavours have yielded multiple academic publications in prestigious journals such as Nature npj Digital Medicine, Stroke, and Journal of Medical Internet Research, amassing nearly 400 citations. Furthermore, she has delivered both poster and oral presentations at international conferences.
Over five years in clinical practice, Dr Wang has honed her expertise in the diagnosis and treatment of prevalent eye diseases, including cataract, glaucoma, retinopathy, and ocular trauma. Her pioneering work in this area underscores her hope and commitment to leveraging cutting-edge technology for the betterment of patient outcomes.