mieducation
Prescribing Steroids in a Post-Flarex Market
Topical corticosteroids represent one of the most potent and efficacious treatments for anterior segment ocular inflammation.11,,22 Possessing variable therapeutic effect, dependent upon active drug potency and penetrance, topical anti-inflammatory preparations elicit therapeutic effect by directly targeting ocular structures.22,,33
Flarex (fluorometholone acetate 0.1%) suspension eye drops were withdrawn from the Australian pharmaceutical market in June 2024, with the remaining supply continuing to be available until it was exhausted. Manufacturer Novartis Pharmaceuticals Australia Pty Ltd cited changes to commercial viability as justification for the discontinuation.4
Thomas Ford considers the implications for clinical practice and the available alternatives for effective management of ophthalmic disease.
WRITER Thomas Ford
LEARNING OBJECTIVES
On completion of this CPD activity, participants should be able to:
1. Recognise topical corticosteroid prescribing trends in Australia,
2. Understand the mechanism of anterior segment inflammation and anatomical barriers to topical drug entry,
3. Compare and contrast the pharmacological properties of topical corticosteroid agents, and
4. Understand key treatment philosophies that underpin the evidence-based management of inflammatory eye disease.
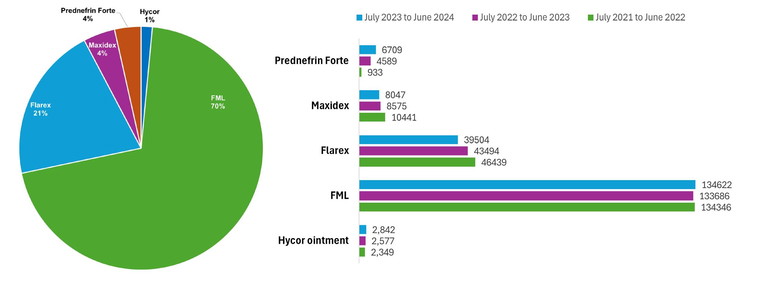
Figure 1. PBS/RBPS topical corticosteroid prescribing by Figure 2. Total number of PBS/RBPS topical corticosteroid prescriptions issued by optometrists between July 2021 Australian optometrists from July 2023 to June 2024.6 and June 2024 per commercially available preparation in Australia.6
Therapeutically endorsed optometrists in Victoria were first permitted to prescribe Schedule 4 topical drugs in 2000 for the management of ophthalmic disease, followed by other jurisdictions.5 Since then, topical corticosteroids have become a key component of the optometrist’s therapeutic arsenal.
Publicly available data from Services Australia indicates the total number of pharmaceutical benefits scheme (PBS) and repatriation PBS (RPBS) prescriptions issued, though does not account for private prescriptions. Figure 1 presents PBS/RPBS topical corticosteroid prescribing data by Australian optometrists between July 2023 and June 2024.6
There was a predominance of FML (fluorometholone alcohol 0.1%) prescribing subsidised by Services Australia during this period, accounting for 71% of PBS/RPBS prescriptions in the 2023–24 financial year, followed by Flarex at 21%. Furthermore, Maxidex (dexamethasone alcohol 0.1%) and Prednefrin Forte (prednisolone acetate 1%, phenylephrine hydrochloride 0.12%) each contributed 4%, with Hycor ointment (hydrocortisone acetate 1%) representing 1% of optometrist PBS/RPBS topical corticosteroid prescriptions in this period. Prescribing trends in terms of number and percentage remained relatively consistent for each preparation over the preceding years, except for Flarex, which demonstrated a 15% decline in prescriptions issued between financial year 2021–22 and 2023–24 (Figure 2).6 FML was removed from the PBS in August 2024.
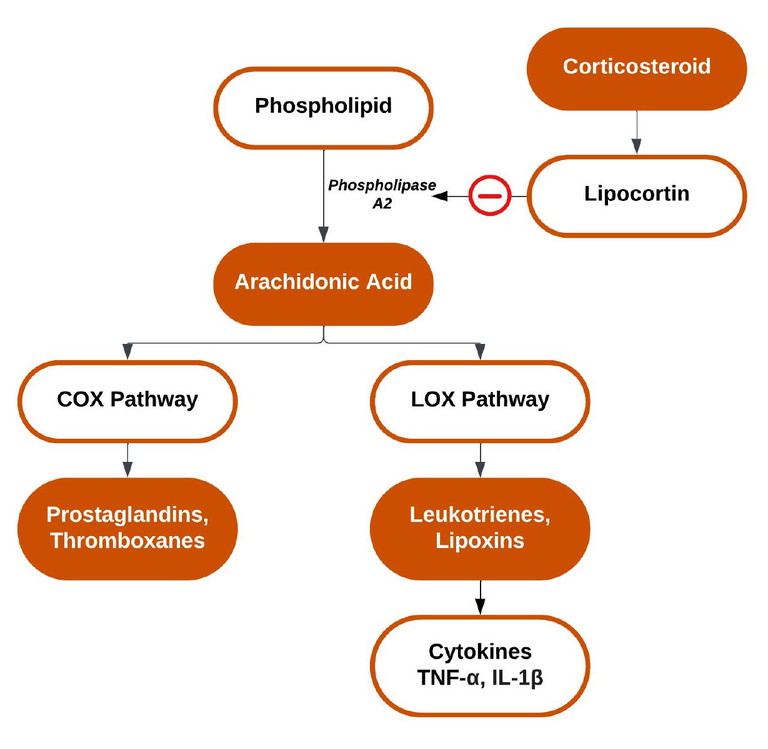
Figure 3. The effect of corticosteroids on part of the inflammatory cascade.
INFLAMMATORY CASCADE
In the ocular context, corticosteroids are indicated predominately in the management of anterior segment pathology via inhibition of the inflammatory cascade.7,8 One part of the inflammatory cascade is characterised by the formation of leukotrienes in response to noxious stimuli.8 This promotes the production of inflammatory mediators such as prostaglandins to injury sites, leading to increased vascular permeability and vasodilation, which gives rise to hyperaemia and oedema, among other sequelae.7 Corticosteroids interrupt this pathway through binding to glucocorticoid receptors, altering gene expression, and upregulating the DNA transcription of lipocortin (Figure 3).9 Lipocortins, in turn, inhibit phospholipase A2, blocking the conversion of phospholipids to arachidonic acid, the precursor to mediators that regulate inflammatory cytokines.7,8
Subsequent inhibition of the inflammatory cascade suppresses prostaglandin analogue and thromboxane synthesis via cyclooxygenase (COX), in addition to leukotriene synthesis via lipoxygenase (LOX).8,10 Mast cell and histamine synthesis is also inhibited by histaminase. Ultimately, this leads to immune suppression and reduced inflammation, given no substrate exists for the COX and LOX pathways to produce inflammatory mediators.8,11
ANATOMICAL BARRIERS
Ocular drug delivery is dependent upon dynamic and static anatomical barriers. The ocular dynamic barrier consists of the tear film, conjunctival cul-de-sac reservoir (inferior fornix), conjunctiva, and episclera; where upon instillation, topical agents are diluted by basal and reflex tears, nasolacrimal drainage, and blinking.11,12 Additionally, the continuous turnover of tear fluid (estimated at 15% per minute) more than doubles following topical drop administration.11-13 This further contributes to the ocular dynamic barrier through the washout effect of the pre-corneal drug, thus reducing its ability to penetrate the ocular static barrier.14
“A core tenet in the prescribing of topical corticosteroids is the relationship between potency and penetrance”
The cornea and sclera primarily comprise the ocular static barrier, where only drugs that pass through the dynamic barrier may penetrate.14 Transcorneal drug transport commences at the lipophilic epithelium consisting of tight junctions, followed by the hydrophilic stroma, which provides low resistance to the diffusion of highly hydrophilic drugs. The lipophilic endothelium, which forms a leaky barrier for passive or active transportation from the stroma to the anterior chamber, also contributes to the ocular static barrier, in addition to two acellular collagenous membranes, Bowman’s layer and Descemet’s membrane.15 As a result, the static barrier tends to reduce drug permeability given impacts to both corneal absorption and penetration, therefore limiting topical drug efficacy. However, this barrier may be overcome by drugs containing enhanced corneal permeability properties.3,14,16 In summary, for a topical corticosteroid to effectively penetrate the cornea, it must be biphasic. That is, both lipophilic and hydrophilic.
TOPICAL DRUG BIOAVAILABILITY
Topical administration constitutes the primary delivery route of ophthalmic corticosteroids for management of anterior segment disorders.2,16 This is advantageous compared to alternate administration routes in terms of ease of instillation, reduced systemic exposure, rapid onset, higher local concentrations, termination of treatment, and lower cost. However, it also poses potential challenges, such as short shelf life, short half-life when administered, poor solubility, the need for frequent instillation, ocular surface toxicity via the drug or preservative, in addition to the risk of systemic absorption and adverse effects compared to non-topical administration.16
One factor, which may pose a challenge to effective anti-inflammatory therapy, is pre-corneal residence time. This refers to the duration of adhesion of the active ingredient(s) to the pre-corneal surface following instillation, which is affected by factors such as ocular physiology, active drug concentration, vehicle, and formulation.3,11 The ocular dynamic barrier acts to rapidly reduce ocular surface residence time and limit drug bioavailability, as a sufficient retention period is required to allow optimal drug penetration.3,11,14,16
Prolonged pre-corneal residence increases ocular bioavailability through sustained drug release. This aids topical drugs to overcome the ocular static barrier, enhancing transcorneal penetration and aqueous humour bioavailability, allowing for ocular surface and/or intraocular therapeutic effect. Subsequently, this allows the active drug to act locally on mucosal surfaces for a greater duration, or to penetrate deeper into ocular tissues to reach the intended target.3,11 One of the most common approaches to prolonging pre-corneal residence time is to increase vehicle viscosity, as aqueous vehicles often demonstrate rapid pre-corneal clearance compared to suspensions and ointments. Increased vehicle viscosity may allow reduced frequency of topical administration, therefore reducing corneal toxicity from preservative-containing preparations while also aiding patient compliance.3,11 In addition to drug pre-corneal residence time, water solubility and the corneal permeability coefficient also impact drug bioavailability within the cornea and aqueous humour, which may be hindered by anatomical dynamic and static barriers.11,16
TREATMENT PHILOSOPHY
The philosophy underpinning the prescribing of topical corticosteroids requires clinicians to consider a balanced regimen: the most appropriate drug dosage to produce the maximum therapeutic effect with minimum adverse effects. Practitioners should consult evidence-based resources and seek expert counsel in instances where their scope of knowledge, confidence or experience is exceeded.
The frequency of topical corticosteroid instillation induces enhanced antiinflammatory response, particularly with Prednefrin Forte.17 However, this does not represent a linear relationship, nor is it true for all preparations. It must also be noted that increased corticosteroid concentration within topical preparations may result in higher intraocular concentrations, but not in all instances.8 As such, an evidence-based approach is required to select the most appropriate corticosteroid formulation, dependent upon specific ophthalmic disorder.
PHARMACEUTICAL FORMULATION
Topical formulations may be prepared as a suspension, solution or ointment in the presence or absence of preservative. Acetates and alcohols form suspensions, whereas solutions are composed of phosphates. Critically, the efficacy of each preparation depends not only on the intrinsic potency of the active drug(s), but also on its penetrance and durability.18
Potency Versus Penetrance
A core tenet in the prescribing of corticosteroids is the relationship between potency and penetrance. Potency is the magnitude of effect produced by a certain dosage or concentration of drug, whereas penetrance refers to the permeability of a drug through ocular membranes.19
Penetrance is dependent upon drug concentration, chemical formulation, and vehicle composition, where greater penetrance allows for lower corticosteroid concentrations to be considered, given its direct therapeutic action at the site of ocular inflammation.2,3 Both potency and penetrance determine drug efficacy within ocular tissues.
Lipophilic acetate bases, such as Flarex and Prednefrin Forte, allow increased binding to glucocorticoid receptors and possess greater penetration of the intact cornea than alcohols, such as FML and Maxidex.20 Therefore, the acetate form of prednisolone penetrates the cornea and enters the aqueous at a greater rate than its alcohol form, leading to an enhanced anti-inflammatory effect within the anterior chamber. Further, suspensions are advantageous in that their properties allow prolonged precorneal resident time, aiding transcorneal penetration and prolonged duration of action.11
“ The efficacy of steroid preparations depend on the intrinsic potency of the active drug(s), in addition to its penetrance and durability ”
Phosphates have the least corneal penetrance compared to acetate and alcohol bases, respectively. Further, phosphate-based preparations demonstrate efficacy in cases of compromised corneal tissue, such as ulcer or abrasion, given the drug is not impeded by an intact lipophilic corneal epithelium. Therefore, greater active drug concentrations may be achieved following epithelial compromise compared to that of an intact cornea. The biphasic cornea may also be affected by inflammatory disease which degrades static lipid barriers, therefore allowing greater phosphate penetrance. Similarly, preservative-containing preparations have greater penetrance than those without, as they disrupt tight junctions between corneal epithelial cells.3
It is well documented that suspensions and ointments possess poor dosage repeatability, dosage accuracy, and inconvenience during administration.16 It is important that suspensions are shaken immediately prior to use, or the administered dosage and therapeutic effect may vary. This is due to the active drug settling towards the bottom of the container while not in use, where the unshaken preparation may contain a lesser proportion of active drug and more vehicle.
PHARMACOLOGICAL PROPERTIES
Flarex Legacy
Flarex was primarily indicated in steroidresponsive inflammatory conditions of the conjunctiva, cornea, and anterior segment. An acetate derivative, Flarex contains lipophilic properties, which enable superior transcorneal penetration compared to the alcohol-based FML. Further, Flarex demonstrates superior efficacy within the corneal stroma, both in the presence and absence of a corneal epithelial defect, compared to Maxidex. Its therapeutic effect was equal to that of Prednefrin Forte for the management of ocular surface inflammatory disease.21,22 The ideal replacement for Flarex is the highly lipophilic Lotemax (loteprednol etabonate 0.2%, 0.5%) due to its comparative risk profile, potency, and penetrance properties within the corneal stroma, where it may reach high concentrations despite a 20-times relative potency difference between Flarex and Lotemax.20,23 As a ‘soft’ steroid, Lotemax also has a lower propensity to elevate intraocular pressure (IOP).17,20
However, like Flarex, Lotemax is commercially unavailable in Australia, accessible only via the Special Access Scheme. Therefore, clinicians must prescribe an alternative that contains sufficient potency and penetrance to elucidate an appropriate therapeutic benefit, while reducing the risk of adverse effects. This requires sound clinical judgement to accurately compare the prescribing of alternate commercially available topical corticosteroid preparations to ensure an appropriate and effective therapeutic response.
FML Versus Maxidex and Prednefrin Forte
While FML is effective within the corneal epithelium, anterior stroma, and episclera, Maxidex exhibits its greatest efficacy within the posterior corneal stroma, and anterior chamber. This is also true for Prednefrin Forte, which achieves greater intraocular concentrations due to its effective penetration of the corneal epithelium.24 While both Maxidex and Prednefrin Forte are potent topical corticosteroids, Prednefrin Forte reaches higher aqueous concentrations (over 20-times the relative concentration of Maxidex) and persists for double the duration, up to 24 hours post-instillation (Table 1).24-26 The mean peak concentration of Maxidex within the aqueous humour is over six-times greater than FML (Table 1, Figure 3). Further, significant concentrations of the active drug, from both FML and Maxidex preparations, remain detectable within the aqueous at four hours and 12 hours, respectively, following administration.1,24,25
Despite FML being 1.6-times the relative potency of Maxidex, poor penetrance of the intact corneal epithelium results in FML having approximately half the corneal stromal therapeutic effect of Maxidex. In instances of epithelial compromise, however, FML demonstrates similar efficacy to that of Maxidex.24,25 Active ingredient concentration may also affect relative potency. Prednefrin Forte contains 10-times the concentration of fluorometholone compared to FML and Flarex, which is merely one factor that contributes to its greater perceived or observed anti-inflammatory effect.24,25
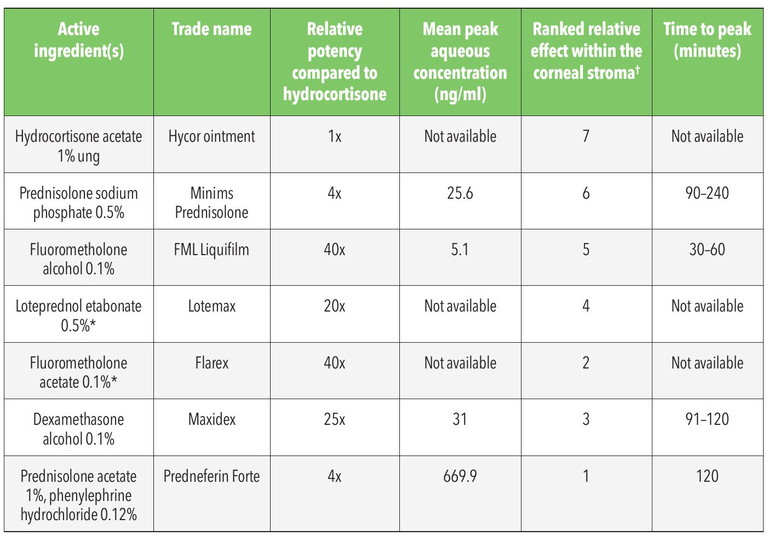
Table 1. Topical corticosteroids approved for prescribing by therapeutically endorsed optometrists in Australia.24-26,29 *Indicates preparations not commercially available in Australia. †Ranking where 1 indicates greatest corneal stromal penetrance with 7 being the lowest.
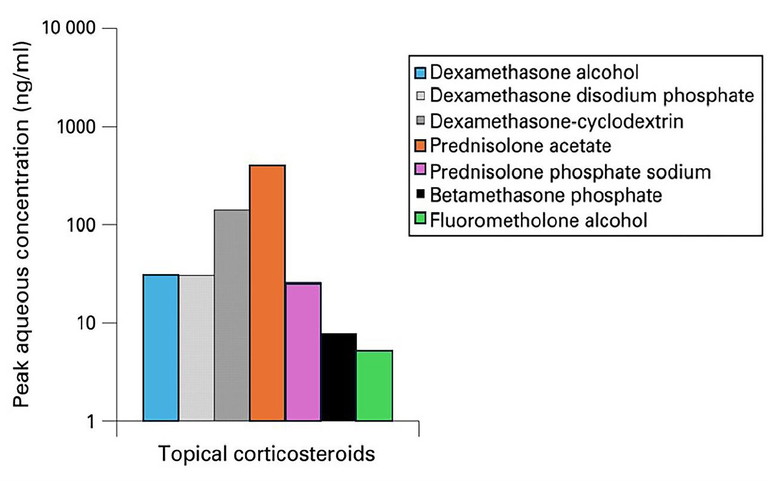
Figure 4. Mean peak aqueous concentration of select topical corticosteroids.1
FML, Maxidex, and Prednefrin Forte all contain the preservative benzalkonium chloride, albeit in differing concentrations, with each available via the PBS except FML, from August 2024. FML and Maxidex are listed within pregnancy category B3, whereas Prednefrin Forte is classified as category C.27,28 Concurrent topical corticosteroid use and contact lens wear is not recommended.
PRESCRIBING DECISIONS IN OCULAR DISEASE
FML is effective within the corneal epithelium, anterior stroma, and episclera, whereas Maxidex exhibits its greatest efficacy within the posterior corneal stroma, and anterior chamber. This is key to guiding the therapeutic management of specific ocular disease.
FML is indicated for moderate to severe ocular surface inflammation, where therapy is expected to be short-term without the need for potent posterior stromal penetrance. However, its low adverse effect profile makes it suitable for use in cases of prolonged ocular surface and anterior corneal disease.27 For example, symptomatic moderate to severe inflammatory dry eye disease may be appropriately managed via a course of FML, as per the Tear Film and Ocular Surface Society Dry Eye Workshop reports.30 FML may also be indicated following metallic foreign body removal from the central anterior corneal stroma to reduce the risk of scarring and subsequent vision degradation, particularly in cases of significant corneal inflammation. Care should be taken to withhold topical corticosteroid administration until reepithelialisation occurs and microbial invasion has been excluded, given the increased infection risk, where concomitant prophylactic anti-microbial agent use is recommended.31-33 Maxidex, however, would be more appropriate in cases of posterior corneal stromal disease given its enhanced penetrance properties.14
Similarly, sterile subepithelial infiltrates present in cases of contact lens-associated red eye may be appropriately managed via FML, in conjunction with prophylactic antimicrobial agents.34,35 It is critical to exclude infectious cause in such instances prior to topical corticosteroid administration given the risk of significant adverse outcomes. In contrast, peripheral stromal infiltrates secondary to marginal keratitis may result in the clinician prescribing antimicrobial agents to limit bacterial colonisation, in addition to a short course of Maxidex.36 In such cases, infiltrative aetiology must be considered prior to commencing treatment, where prescribers should proceed with caution in the presence of an epithelial defect.
Post-surgical inflammation following cataract or refractive laser procedures tends to result in the prescribing of Maxidex, however, post-corneal graft and bouts of acute anterior uveitis indicate the use of preparations with greater penetrance, such as Prednefrin Forte.5,14,36,37 The penetrance properties of Prednefrin Forte are also of benefit in severe, central bacterial keratitis, and stromal or endothelial herpes simplex keratitis, as described in the SCUT and HEDS trials, respectively.31,32,38 However, antimicrobial agents remain the mainstay of topical therapy in both ocular diseases.
“The lower the anti-inflammatory potency of the topical agent, the lower the rate and magnitude of subsequent IOP response”
TAPER VERSUS TITRE
Prolonged corticosteroid use may result in adrenal suppression, reducing the production of natural cortisol by the adrenal gland and mature circulating leukocytic elements.8 As such, a titre or taper regimen is required. Titre refers to the concentration change of a drug during active disease, whereas taper refers to drug concentration change in absent or resolved disease. Despite topical corticosteroid administration for ≤14 days not ordinarily requiring a titre or taper regimen in adults, it may be beneficial to ensure that inflammation has been adequately controlled.8 A titre or taper strategy is, however, necessary in circumstances where treatment exceeds 14 days as abrupt cessation of corticosteroids may result in rebound inflammation via the proliferation of immature cells. This refers to an inflammatory response towards the minimal residual antigen within the affected tissue, often accentuated despite the low proportion of remaining antigen present. While this is more common following systemic corticosteroid use, cases have been documented in relation to topical ocular corticosteroid administration.39
ADVERSE EFFECTS
There are extensive complications associated with topical corticosteroid use, the most common of which are described below. Despite these potential effects, topical delivery is often preferred to systemic administration to reduce risk profile in most anterior segment disease.
Elevated IOP secondary to reduced prostaglandin production, reducing trabecular meshwork metabolism and thus aqueous outflow.2,8 This may be observed in as little as 14 days of continuous use in adults, but most commonly within 4–12 weeks. In children, this period reduces to eight days.1,40 Of the commercially available topical corticosteroids, Maxidex has the greatest propensity to elevate IOP, with FML being among the least likely due to its poor transcorneal penetration.41 Steroid response has been demonstrated in 60% of the population by an IOP increase of <6 mmHg following topical corticosteroid installation, 6–15 mmHg in 35% of cases and >15 mmHg in 5%. This mechanism is reversible, with acute steroid responses resolving within an average of three days post-steroid cessation, with chronic IOP elevation returning to <21 mmHg within one to four weeks.42,43 In principle, the lower the antiinflammatory potency of the topical agent, the lower the rate and magnitude of subsequent IOP response.43
Rebound inflammation following a rapid or absent tapering strategy, or in cases where topical corticosteroid concentration is insufficient for the degree of inflammation.39
Delayed corneal wound healing should topical corticosteroids be administered following epithelial compromise. This is exacerbated in cases of concomitant topical non-steroidal anti-inflammatory drug (NSAID) use, where a synergistic therapeutic effect has been reported to enhance NSAID penetration.44 This is particularly pertinent in resolving macular oedema following postoperative intraocular inflammation, where corneal epithelial compromise increases the ability of active drug(s) to reach the retina and choroid.45,46
Increased susceptibility for opportunistic infection given corticosteroid-induced immune suppression. Hence, the contraindication of corticosteroid use during active herpetic infection without concurrent oral antiviral use, given the risk of reactivation or exacerbation of the herpetic disease process.2 Corticosteroids may also mask, delay, or exacerbate the presentation of secondary viral, fungal, or bacterial infections.17
Posterior subcapsular cataract (PSC) secondary to the oxidation of lens proteins, following the covalent binding of corticosteroids.2 While PSC has been reported following ≥4 months of continuous corticosteroid administration, it is more common following 12 months of high dosage use. PSC does not progress when corticosteroids are discontinued, and may be partially reversible upon cessation.2
“Given the potential adverse effects of topical corticosteroids, clinicians must balance the risks by managing each case of ophthalmic disease with the most appropriate preparation, dosage, and frequency of topical ocular corticosteroid administration”
Given the potential adverse effects of topical corticosteroids, clinicians must balance the risks by managing each case of ophthalmic disease with the most appropriate preparation, dosage, and frequency of topical ocular corticosteroid administration. This is to ensure appropriate anti-inflammatory efficacy, while reducing the need for prolonged corticosteroid usage. It is pertinent for optometrists to proactively manage the risk of elevated IOP by performing appropriate IOP monitoring prior to, and during, the prescribing of topical corticosteroids, and actively consider the risk of rebound inflammation, where an appropriate taper or titre regimen depends upon specific disease process.
Clinicians may opt for a binary taper regimen in cases of short-term topical corticosteroid use, where the reduction in dosage occurs over a specific duration (i.e. QID for four days, TDS for three days, BD for two days, QD for one day). In contrast, a more prolonged regimen may be employed in complex ocular disease such as posterior uveitides, with the reduction of one topical corticosteroid drop per month.17

Thomas Ford BMedSc (VisSc) MOptom AdvCertGlauc is a therapeutically endorsed optometrist with George and Matilda Eyecare. He has experience in private ophthalmology and has completed further studies in paediatric eyecare and glaucoma. Interests include the therapeutic management of ophthalmic disease and myopia control, where he frequently co-manages complex cases with ophthalmology. In 2024, Mr Ford was awarded the South Australian Rural Health Award for outstanding clinical excellence and patient care.
CONCLUSION
Corticosteroids play an important role in the effective management of ophthalmic disease. While they possess well-documented anti-inflammatory properties, topical ocular corticosteroids are associated with risk of adverse effects. It is therefore incumbent upon the prescribing clinician to not only be aware of the therapeutic actions of drugs at their disposal, but also the evidence relating to ocular drug toxicity and complications. Critical too, is that clinicians remain cognisant of the bounds of their clinical knowledge and competencies, seeking expert counsel when necessary to ensure appropriate and safe clinical practice.
To earn your CPD hours from this article visit: mieducation. com/prescribing-steroids-in-a-post-flarex-market.
The author wishes to acknowledge Shazaan Khambiye, Professional Services Manager, George and Matilda Eyecare, and Dr Alex Hui, School of Optometry and Vision Science, University of New South Wales, for review of this article.
Production of this article was sponsored by AbbVie. The author has no relevant disclosures.
References available at mieducation.com.