mieducation
Red Light on the Horizon
Ophthalmologists’
Perspectives on RLRL
In this article, Eyerising International’s Dr Yuri Aung hears from four leading ophthalmologists from around the world – Dr Loren Rose (Australia), Professor Jason Yam (Hong Kong), Mr John Bolger (United Kingdom), and Professor Junwen Zeng (China) – as they share their expert perspectives on repeated low-level red-light therapy (RLRL) for myopia with case studies from a range of difficult-to-treat patients.
WRITERS Dr Yuri Aung, Dr Loren Rose, Professor Jason Yam, Mr John Bolger, Professor Junwen Zeng
LEARNING OBJECTIVES
On completion of this CPD activity, participants should:
1. Understand the latest evidence behind repeated low-level red-light (RLRL) therapy for myopia control,
2. Be able to identify patients that would be appropriate to start RLRL, and
3. Know how to monitor and follow up patients on RLRL.
Over the past two decades, the global burden of myopia has risen with unprecedented speed. Over half of the world’s population is projected to be myopic by 2050, with a significant proportion developing high myopia and its associated complications.1 Amid this growing public health concern, the race for effective myopia control interventions has intensified.
Today, eye care practitioners can offer families a growing suite of options: low-dose atropine, orthokeratology (OK), as well as a range of multifocal soft contact lenses and myopiacontrol spectacle designs. One newer addition to the myopia management toolbox, and indeed in its own category entirely as a medical device, is repeated low-level red-light therapy.
RLRL is delivered through a home-use desktop device that emits visible red light at 650 nm through laser diodes. Patients use it for three minutes, twice daily, five days per week. RLRL is presumed to work by increasing choroidal blood flow and inducing choroidal thickening, possibly alleviating the scleral hypoxia implicated in myopia progression. Furthermore, it has been shown not only to slow axial elongation but in some patients, to cause significant axial shortening.2
In recent years, a growing body of research has validated the clinical efficacy of RLRL. The landmark randomised controlled trial published in Ophthalmology in 2021 by Professor Mingguang He and colleagues first demonstrated a 69% reduction in axial elongation progression over 12 months compared to controls, with no significant adverse events reported.3
Subsequent studies from China for up to two years of use have supported these findings, with particular effect seen in highly myopic populations with mean axial shortening observed.4 International data has also begun to emerge, with three-month results published from Australia showing mean axial shortening,5 and 12-month data from Australia, Japan, and Spain presented at international conferences.6 Indeed, RLRL was recognised as the highest-effect intervention in the recent living Cochrane review for myopia control,7 although given the novelty of this treatment, the evidence remains of very low certainty. Nevertheless, RLRL is increasingly generating discussion as a highly effective and noninvasive option for myopia control.
As with any new intervention, safety is also of paramount importance. A systematic review evaluating the safety of RLRL identified that of all myopia control interventions, it had the lowest side effect incidence of 0.088 per 100 patient years, with no adverse events reported from any of the published clinical trials of RLRL.8 In fact, it identified only one case in the literature, reporting reversible retinal changes to the ellipsoid zone.
A separate hot topic in RLRL has been the recent reclassification to Class III in China,9 placing it on par with orthokeratology lenses. This move by the National Medical Products Administration reflects a broader effort to improve quality standards and enhance safety, efficacy, and long-term credibility of RLRL. Until manufacturers can meet the heightened standards, existing users have been permitted to continue using RLRL and existing devices to be sold, but further manufacture and sale of devices in China is not permitted. Notably, this does not impact the international regulatory approvals of RLRL, where the Eyerising Myopia Management Device is the only RLRL device thus far to have been approved and commercially sold in various international jurisdictions.
In this article, four ophthalmologists expand on their global experiences with the Eyerising RLRL device.
First, Dr Loren Rose shares her insights from early clinical adoption in Australia, expanding on a two-year case study previously resistant to other myopia treatments. Prof Jason Yam from Hong Kong draws on his extensive research experience to discuss how RLRL may fit into myopia management, and shares a case study of a patient he had titrated up to 1% atropine before successfully transitioning to RLRL. Mr John Bolger from the UK discusses his transition from sceptic to convert through first-hand experience with RLRL on his treatment-resistant patient. Finally, Prof Junwen Zeng discusses his ongoing multi-year real-world study, the implications of the China reclassification, and presents a four-year case study illustrating the long-term efficacy and safety of RLRL beyond what has been experienced in the Western world.
AUSTRALIA: DR LOREN ROSE
Case Study
Jay Cho,* a 10-year-old child of Asian descent, was first reviewed in late 2020. He presented with glasses for myopia right eye (OD) spherical equivalent refraction (SE) -2.5D, left eye (OS) SE -2.00D, and right axial length (AL) 25.13 mm and left AL 24.98 mm, with a documented fast progression of >0.2 mm/one year. Treatment with atropine 0.01% and single vision glasses had already been initiated. At that visit, his dose of atropine was increased to 0.05%, and his glasses were upgraded to right SE -3.5D and left SE -3.0D.
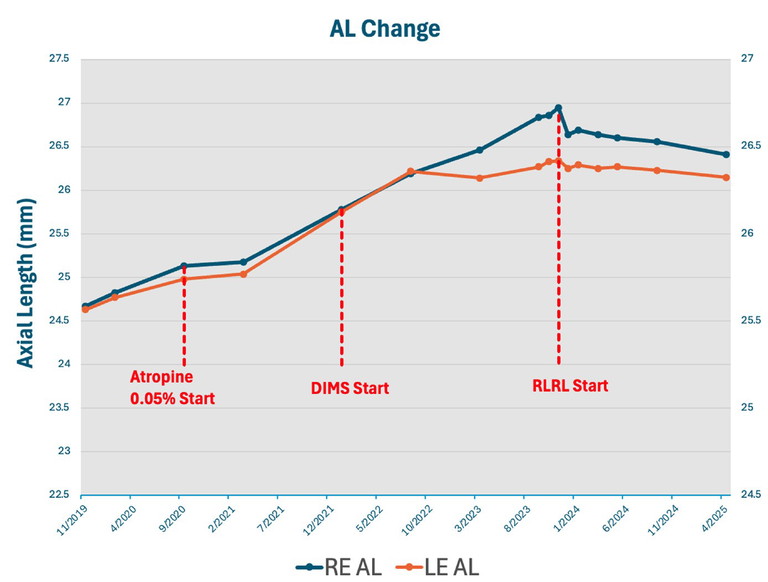
Figure 1. Change in axial length in the case study for Jay Cho* from November 2019 to April 2025.
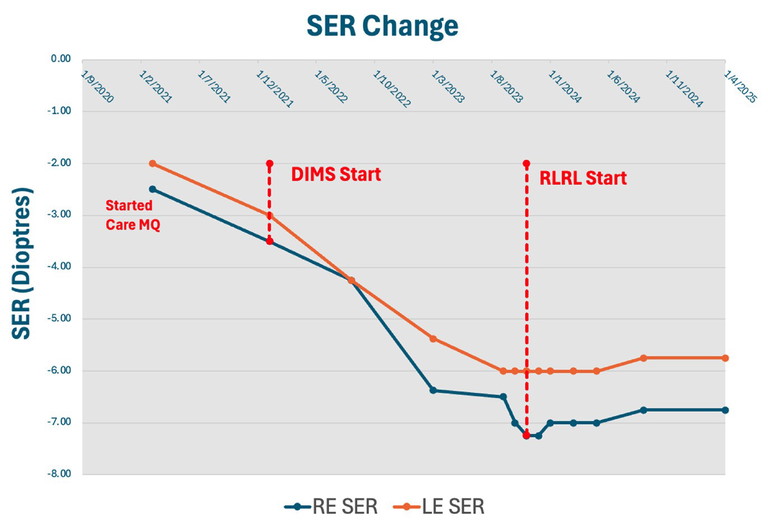
Figure 2. Spherical equivalent refraction (SER) of the same patient over the period September 2020 to April 2025.
At six-month follow-up, his progression initially reduced to 0.05 mm and 0.06 mm. However, although compliance with the drops was good, the family did not present for the next six-month follow-up. Just over two years after initial presentation, his refraction had increased to SE each eye -4.25D, and defocus incorporated multiple segments (DIMS) lenses were prescribed. Unfortunately, Jay continued to progress quickly while on combination DIMS and atropine 0.05% for the following 18 months, with an axial growth rate of the right eye on average 0.33 mm/six months. His refraction at this point was right SE -7.0D and left SE -6.0D.
In September 2023, the patient and family consented to start RLRL. The protocol was discussed, and initiation of RLRL began with discontinuing the atropine drops and monthly monitoring of pupil size and axial growth. Once the pupil was reduced to physiological size (4 mm) and reactivity after two months, RLRL was started. Remarkably, Jay complied very well with the treatment, with a compliance rate of 90.47%.
As this was one of my first patients on RLRL, the patient was followed monthly for the first three months with visual acuity testing, axial measurement, and optical coherence tomography (OCT), including choroidal thickness, which was compared during atropine washout and baseline to postRLRL. After the initial three months, he was reviewed again three months later and then every six months onwards.
Jay has now completed over 18 months of treatment. At his recent 18-month review, he had refraction of right SE -6.75D and left SE -5.75D, a reduction of 0.25D over the 18 months, which is fantastic given his previous quick progression. His AL had also reduced by right 0.54 mm and left 0.19 mm with corresponding choroidal thickening.
Hence, he has had virtually no myopic progression since beginning RLRL treatment. Best corrected visual acuity has been maintained at 6/6 in both eyes, and the patient only reported mild transient afterimages with no other sequelae.
Perspective of an Early Adopter
High progressive myopia that is resistant to a combination of peripheral defocus glasses and low-dose atropine (0.05%) has limited treatment options. This case highlights the effectiveness of RLRL as a newer player in controlling progression in these cases.
Safety is paramount with any new intervention, which in my case included close monitoring before starting RLRL. I also ensured an adequate washout period from atropine where the pupil size returned to physiologically normal size before starting RLRL, as combination use of RLRL and atropine is contraindicated. The patient was also closely monitored in the first few months. This monitoring was completed via vision and OCT imaging at monthly intervals, which was slowly extended during treatment.
I found that the regular follow-up schedule was also helpful in discussing tolerability with the patient and any potential side effects, beyond the primary goal of ensuring continued macular health and vision preservation. In particular, the patient and family were counselled to report any extended afterimages beyond five minutes as per the protocol, as this may indicate a potential overt response to the treatment, which can then be followed up with an assessment for any macular changes. Fortunately, this has not been the case for any of my patients thus far. I also found compliance particularly easy to monitor, as rather than self-reported compliance, the patient could log into the treatment portal and show me a quick summary of their monthly compliance levels.
Going forward, for my highly myopic patients with fast progression despite combination treatment, I will discuss and consider their options to change treatment, stop low-dose atropine, and initiate RLRL.
I would also recommend such cases to my fellow clinicians who are seeking to identify their first patient suitable for RLRL. Observing these results first-hand in a complex patient of mine provided some considerable degree of confidence in the treatment’s effect.
HONG KONG: PROF JASON YAM
Case Study
Jing Kwok* was first referred to my clinic at age eight with documented progressive myopia in late 2020. At baseline, her refractive error was -3.25D with axial length of 23.69 mm. We first attempted 0.05% atropine OD, which was increased in 2021 to three times daily and by 2022 to four times daily. However, she experienced continued myopic progression and by August 2022, her refractive error had jumped to -4.5D and axial length of 24.64 mm. This axial elongation trajectory was particularly worrisome given her young age and the risk of eventual pathological myopia.
Given her continued progression, Jing was started on 1% atropine in December 2022. Although 1% atropine has known side effects, I wanted to determine whether this high concentration would be sufficient to slow her myopic progression, which had concerningly risen to 0.57 mm/year, well above the threshold for stable myopia.
Jing continued on 1% atropine for another year and a half. However, even 1% atropine was not effective at slowing her rapid myopic progression. By March 2024, high myopia had onset as her refractive error was -6.5D and axial length 24.95 mm. Therefore, alternative therapy was warranted.
During this period, I had begun my ongoing research into RLRL in collaboration with Prof He and thought that RLRL may be an option. Although it was newly introduced in Hong Kong, I had a thorough discussion with Jing and her family about using RLRL as an alternative treatment and the evidence base thus far. From a risk-benefit analysis, atropine was clearly not working well for Jing and with RLRL showing such strong efficacy in high myopia, the family consented. She was weaned off of atropine and started on RLRL in March 2024.
Once RLRL was initiated, Jing’s myopic progression slowed noticeably. In fact, at each appointment, her refractive error not only stabilised but improved, from a 0.25D improvement in the first month to an overall 0.75D improvement in nine months. This was coupled with overall axial length shortening, with a significant nine-month axial length shortening of 0.18 mm. For Jing and her family, this was fantastic news as her current refractive error was -5.75D, now below the threshold of high myopia. Furthermore, this outcome was particularly significant because Jing’s axial length trajectory prior to RLRL suggested a high-risk profile for rapid progression and possible future degenerative changes. No adverse events were reported, and compliance remained high throughout the treatment period.
Clinical Potential of RLRL
Managing progressive myopia in paediatric patients remains a significant clinical challenge, particularly in cases resistant to conventional treatments. This case presents an example of how RLRL may be a timely change for those who are not responding well to atropine treatment. In fact, it underscores the potential future paradigm shift in myopia management, where RLRL may be a mainstay in those unresponsive to pharmacological or optical interventions. I have since made this transition in a number of my treatmentresistant patients and seen similar effects.
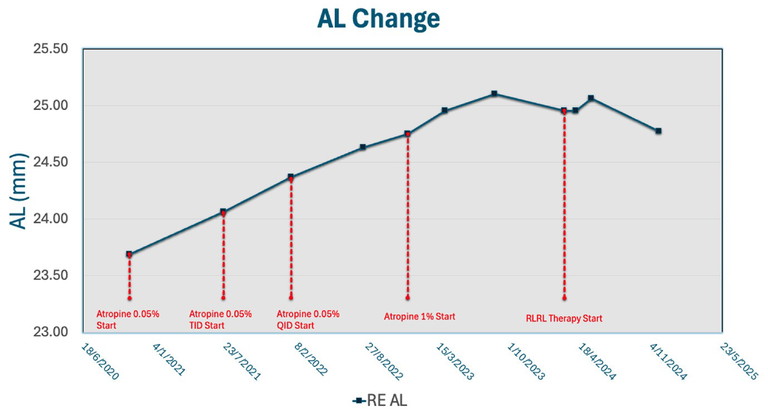
Figure 3. Axial length changes for patient Jing Kwok* from mid-2020 to late 2024.
As myopia prevalence continues to rise globally, integrating RLRL into mainstream clinical care could help us preserve vision and prevent future ocular complications in countless children like Jing. Its relevance to future myopia management should not be underestimated.
UK: MR JOHN BOLGER
The Transition from Sceptic to Convert
When I first heard of RLRL therapy, I must admit I was sceptical that such a modality could produce the results claimed, particularly the unheard-of axial shortening. It seemed far-fetched that something as non-invasive and simple as shining a light on the fovea would produce such marked effect. Additionally, I was concerned about the safety of such a process on the developing eye, and how substantial the afterimage may be.
However, as time went on, more and more reports in prestigious scientific journals all seemed incredibly positive. This was coupled with continued reports of significant axial shortening on RLRL achieved in Chinese populations with well-known high rates of myopic progression.
At the same time, I was encountering more children who were not stabilising in their myopia progression despite being on the maximum anti-myopia therapies I could prescribe. These included children on combinations of 0.05% atropine, anti-myopia glasses or contacts, and considerable lifestyle modifications to achieve more sunlight exposure, that were still progressing at 0.35 mm/year and more. I knew these children were heading for high and pathological myopia by adulthood.
“... all my patients using RLRL have shown a similar dramatic reduction in their rates of myopic progression, and none have experienced any side effects”
I therefore began introducing RLRL to these families, offering the opportunity to try this newer treatment since nothing else had worked. I counselled them thoroughly on the risk-benefit of this decision, acknowledging the knowledge gaps with this newer treatment, but suggesting that whatever unknowns of this novel treatment may be no worse or indeed preferable to the risks of high myopia.
Case Study
My first patient was a nine-year-old White girl with high myopia. She had been managed with OK and 0.05% atropine; however, after one year, her axial length had increased by 0.45 mm in her right eye and 0.52 mm in her left eye. Given this marked progression, we discussed switching to RLRL, which she and her parents consented to, and carried out some more detailed retinal scanning including angio OCT with the Revo from Optopol. On commencing RLRL, her axial length was 25.43 mm in her right eye and 25.45 mm in her left.
At her one-month review, it was reassuring to see no change on OCT, and more importantly, no significant change in her axial length. In fact, at two months into treatment, her right axial length had decreased by 0.06 mm and her left by 0.03 mm. She was not troubled by any afterimage, and found she was able to resume normal activities immediately after RLRL sessions.
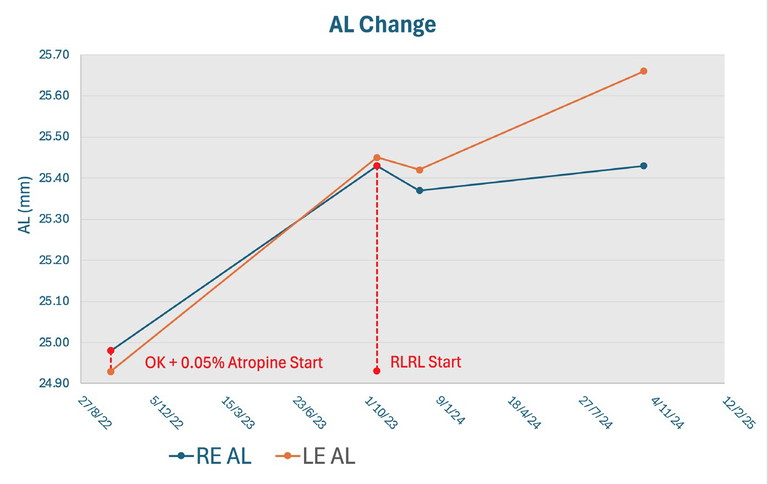
Figure 4. Axial length changes for a nine-year-old patient with high myopia, treated initially with OK and 0.05% atropine, before switching to RLRL.
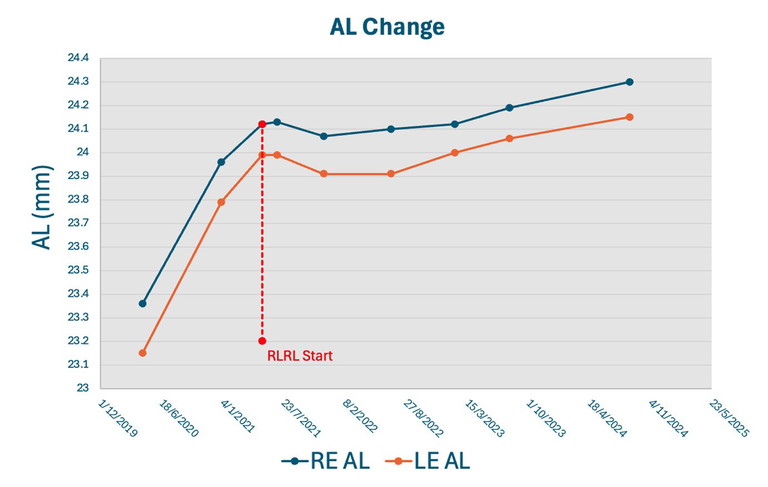
Figure 5. Axial length change of a real-world Chinese case study receiving RLRL treatment.
Further follow-up at six months and 12 months continued to be positive. At 12 months, her axial length had shown no progression. In fact, her right eye had returned to her baseline at the start of treatment and her left eye had only minimal progression of 0.2 mm, which was still much better controlled than before starting RLRL. I was impressed by this, as this was a child who again had been progressing rapidly on combination treatment prior. As much as I had read of axial shortening in the literature, it was gratifying to directly observe its occurrence. The patient has now been on RLRL for approaching two years and has been tolerating it very well with a compliance rate of 80–90%. We will soon have her twoyear follow-up to determine whether to halt treatment or continue.
Since this initial experience, my threshold for starting patients on RLRL has become much lower. I am, in fact, likely to advise RLRL in patients with rapid axial length elongation, not only for those who have been treatment-resistant but especially so in these cases. Although my patient numbers on RLRL remain small, all my patients using RLRL have shown a similar dramatic reduction in their rates of myopic progression, and none have experienced any side effects. It has become a significant part of the treatment we offer, and I envisage a similar transition happening to my fellow clinicians as they too experience first-hand results.
CHINA: PROF JUNWEN ZENG
From Research to Real World
As both a clinician and researcher, I have had the rare opportunity to follow the evolution of RLRL from its early clinical trials to real-world practice, beginning with my initial research with Prof He. Since then, I have launched one of the world’s largest longitudinal studies on RLRL in children, which now has over 2,600 children recruited. This is also the longestrunning RLRL safety dataset globally, with OCT examinations of these patients not identifying any retinal changes thus far.10
“Ultimately, I believe that as was the case with orthokeratology, we will soon reach a tipping point in global understanding and acceptance of RLRL”
China is unique in that RLRL has been approved for many years, and so in my clinic, I often encounter patients who have been on RLRL beyond the two years that it has been introduced internationally. It has been encouraging to see the multi-year results continue to be strong, with high treatment compliance maintained as well.
Having discussed RLRL with my colleagues overseas, I do understand some of their initial scepticism with RLRL, often due to unfamiliarity with the modality but also a sense of the results being almost ‘too good to be true’. A key issue they often note is long-term safety, which I hope my multi-year real-world results in China will address, as will emerging data from international studies that are underway.
The reclassification of RLRL devices in China to Class III also understandably raised concerns. However, I must acknowledge the nuances of China’s National Medical Products Administration compared to other countries, which previously allowed RLRL devices to be approved on a provincial level as Class II devices and resulted in many different brands flooding the market with questionable efficacy and safety data. In this regard, the reclassification may even be a positive step forward for greater mainstream consideration and acceptance of RLRL, as higher regulatory scrutiny will distinguish between evidence-based RLRL technologies and lessproven alternatives. Ultimately, I believe that as was the case with orthokeratology, we will soon reach a tipping point in global understanding and acceptance of RLRL.
Case Study
For my case study, I have chosen a patient I have treated for the past four years with RLRL. When this boy first presented at age seven, he already had an axial length of 24.12 mm OD and 23.99 mm OS with a spherical equivalence of -1.875D OD and -1.25D OS. He had tried low-dose atropine but had struggled with rapid axial length elongation of 0.76 mm OD and 0.84 mm OS in the past year and approximately 1D spherical equivalence progression. Given his concerning trajectory, we opted to switch to RLRL.
In his multi-year follow-up since, the patient has experienced no side effects or prolonged afterimage, preserved his best corrected visual acuity, and observed no changes on OCT.
However, what I wanted to highlight in this case is the stability of his ocular biometrics over time. Over three years of consistent therapy, after an initial period of axial shortening for the first six months, his axial elongation remained minimal.
Indeed, in August 2024, his axial length was 24.30 mm OD and 24.15 mm OS – a total increase of only 0.18 mm OD and 0.16 mm OS in more than three years. This translates to an average annual elongation rate of approximately 0.06 mm/year, which is well below the typical progression rate of Chinese children of his age, which can often exceed 0.3 mm/year and markedly below his initial progression on atropine of 0.8 mm/year. Just as crucially, his spherical equivalence had even improved by +0.125D in both eyes.
To me, this case underscores several critical points. Firstly, multi-year RLRL use is not only feasible but safe and effective. Secondly, initial axial shortening may be observed with RLRL as in this child, and particularly in highly myopic children. Moreover, it is important to consider the consistent slowing of axial elongation observed with RLRL even after axial shortening subsides. The durable suppression of axial elongation observed in this child is often observed in my clinic. Similarly, in my real-world study, it is this markedly reduced multi-year rate of axial length change that I want to better understand, benchmarked against typical progression rates in Chinese populations, as I believe it speaks to RLRL’s greater long-term potential.
THE FUTURE OF RLRL
Taken together, these case studies present early clinical observations from ophthalmologists across diverse myopia management contexts. They offer valuable insights into identifying patients who may benefit most from RLRL, including those resistant to conventional treatments.
As these multi-year frontline experiences show, RLRL is emerging as a promising addition to the myopia management toolbox, with the potential to reshape how we approach myopia control in the years to come.
Patient names changed for anonymity.
This article was sponsored by Eyerising International.
To earn your CPD hours from this article, visit mieducation.com/red-light-on-the-horizonophthalmologists-perspectives-on-rlrl.
References
1. Holden BA, Fricke TR, Resnikoff S, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016 May;123(5):1036-42. doi: 10.1016/j.ophtha.2016.01.006.
2. Wang W, Jiang Y, He M, et al. Clinically significant axial shortening in myopic children after repeated low-level red light therapy: A retrospective multicenter analysis. Ophthalmol Ther. 2023;12(2):999-1011. doi: 10.1007/s40123-022-00644-2.
3. Jiang Y, Zhu Z, He M, et al. Effect of repeated low-level red-light therapy for myopia control in children: A multicenter randomized controlled trial. Ophthalmology. 2022 May;129(5):509-519. doi: 10.1016/j.ophtha.2021.11.023.
4. Liu G, Liu L, Wei R, et al. Axial shortening effects of repeated low-level red-light therapy in children with high myopia: A multicenter randomized controlled trial. Am J Ophthal. 2025 Feb;270:203-215. doi: 10.1016/j.ajo.2024.10.011. Epub 2024 Oct 16.
5. Deen N, Zhu Z, He M, et al. Three-month interim analyses of repeated low-level red-light therapy in myopia control in schoolchildren: A pilot multi-ethnic randomized controlled trial. Ophthalmic Epidemiol. 2025 May 19;1-9. doi: 10.1080/09286586.2025.2500020. Epub ahead of print.
6. Japan: Igarashi-Yokoi T. APAO 2025. Australia: Deen, N. APSPOS 2025. Spain: Fernandez-Velazquez, F. EVER 2025 [Upcoming].
7. Lawrenson JG, Huntjens B, Walline JJ, et al. Interventions for myopia control in children: a living systematic review and network meta-analysis. Cochrane Database Syst Rev. 2025 Feb 13;2(2):CD014758. doi: 10.1002/14651858.CD014758.pub3. Update in: Cochrane Database Syst Rev. 2025 Feb 13;2:CD014758. doi: 10.1002/14651858.CD014758.pub3.
8. Chen Y, Xiong R, He M, et al. Safety of repeated low-level red-light therapy for myopia: A systematic review. Asia Pac J Ophthalmol (Phila). 2024 Dec;13(6):100124. doi: 10.1016/j.apjo.2024.100124.
9. mivision, Red light therapy regulations change in China [Internet] 2015, Feb 17. Available at mivision.com.au/2025/02/red-light-therapy-regulations-change-in-china [accessed June 2025].
10. Wang, L. Poster presentation at the Association for Research in Vision and Ophthalmology 2025 annual meeting.

Dr Yuri Aung MBBS BSc (Hons) is the Director of Clinical and Regulatory Strategy at Eyerising International, where she coordinates their ongoing global research. She holds a medical degree and an intercalated degree in healthcare management from Imperial College London, and has published numerous peer-reviewed publications.

Dr Loren Rose BSc (Hons I) MBBS (Hons) PhD FRANZCO is a clinical senior lecturer at Macquarie University and an Adjunct Associate Professor at the University of Canberra. She practises privately at Sydney Eyecare Burwood, Sydney. In 2021, she completed her PhD, titled Myopia in Children, at Macquarie University.

Professor Jason Yam MBBS MD MPH FRCSEd FCOphthHK FHKAM is Professor and Undergraduate Division Head at the Department of Ophthalmology and Visual Sciences, Chinese University of Hong Kong. He is the recipient of the International Myopia Conference Josh Wallman Memorial Lecture Award 2022, and Asia-Pacific Academy of Ophthalmology De Ocampo Lecture Award 2023. In 2024, he received the National Science Fund for Distinguished Young Scholars to support his myopia research project for the next five years.

Mr John Bolger MB BCh BAO DO FRCS FEBOS-CR is a consultant ophthalmologist and surgeon specialising in myopia control for children, cataract and refractive laser eye surgery. He is the Director of an independent eye care practice, My-iClinic in London, and has lectured extensively in the UK and abroad.

Professor Junwen Zeng MD PhD is Professor and Chairman of the Refraction and Low Vision Department at Zhongshan Ophthalmic Center of Sun Yat-sen University in Guangzhou, China. His clinical and research focus as principal investigator is on myopia prevention and treatment, where he has published over 90 peerreviewed articles.