mi education
mieducation
Treating Dry Eye with Ciclosporin 0.09% Practical Guidance for Eye Care Practitioners
Eye care practitioners are likely to spend an increasing proportion of their time managing patients with dry eye disease (DED) due to the risk of DED increasing with age and an ageing population in general.
The impact of DED on vision, quality of life, and work productivity is considerable, as is the economic burden of the disease, especially indirect costs due to reduced work productivity.1
Here, the authors discuss the use of ciclosporin 0.09% as a treatment option for moderate-to-severe DED, and advise that patient education is key.
WRITERS Dr Amy T. Wang and Dr Elias Kehdi
LEARNING OBJECTIVES
On completion of this CPD activity, participants should be able to:
1. Understand why treating underlying inflammation is important in moderateto-severe dry eye disease for long-ter
2. Understand the role of ciclosporin 0.09% in the management of moderateto-severe dry eye disease,
3. Appreciate the practical considerations including patient education in ciclosporin 0.09% management therapy,m disease management,
4. Understand how to use ciclosporin 0.09% for moderate-to-severe dry eye disease in clinical practice for best patient outcomes.
WRITERS Jason Holland, Dr Margaret Lam, Dr Leigh Plowman, Daniel Tracton, Hugh Bradshaw, and Jason Teh.
Dry eye disease (DED) is a disorder of the ocular surface involving several pathological processes, including inflammation, which can result in ocular surface damage.
DED, also known as keratoconjunctivitis sicca, is a condition commonly encountered by ophthalmologists and optometrists, reflecting its high prevalence (5–50% of the global population).1
Symptomatically, DED is primarily characterised by ocular discomfort and visual disturbances.2 The terms dryness, redness, itchiness, grittiness, burning, and foreignbody sensation are variously used by patients to describe ocular discomfort, which is the cardinal feature of the disease. 2,3 Ocular discomfort tends to be worse on waking.
The important clinical signs of inflammation include corneal staining, conjunctival staining, and reduced tear meniscus height (TMH).
PATHOPHYSIOLOGY
DED has multiple causes and risk factors that lead to loss of tear film homeostasis and the subsequent initiation of biological processes that are detrimental to the ocular surface. 4
Tear hyperosmolarity is the primary pathophysiological event in DED.2,5 It is caused by excessive evaporation of the tear film, i.e., evaporative dry eye (EDE), reduced tear lacrimal gland production, i.e., aqueous deficient dry eye (ADDE), or possibly a combination of the two. 5,6
EDE involves lid-related causes, e.g., meibomian gland dysfunction (MGD), anterior blepharitis, infrequent blinking, and ocular surface-related causes, e.g., contact lens wear, allergic eye disease.5,6 ADDE is due to lacrimal gland dysfunction, e.g., Sjögren’s syndrome, age-related dry eye.
Although the majority of DED cases are the EDE subtype, one-third of patients presenting with signs of both EDE and ADDE in a clinic-based cohort suggests that the two subtypes exist as a continuum.7
ROLE OF INFLAMMATION
Tear hyperosmolarity can damage the ocular surface directly or indirectly by triggering an innate inflammatory response involving ocular surface epithelial cells. 4,6 T cell lymphocytes play a key role in the inflammatory process, including an adaptive T cell immune response following the initial innate immune response.
Tear hyperosmolarity is thought to launch a cascade of signalling events within surface epithelial cells, which results in the release of inflammatory mediators.6 These mediators, together with the tear hyperosmolarity itself, cause damage to the ocular surface. Specifically, the inflammatory mediators induce apoptosis of conjunctival epithelial and goblet cells, corneal epithelial cells, and lacrimal gland epithelial cells as well as neurosensory impairment of lacrimal gland function. The damage is reinforced by inflammatory mediators from activated T cells, recruited to the ocular surface.
These events also cause tear film instability leading to early tear film break-up, which amplifies tear hyperosmolarity. Collectively, these biological processes constitute a selfreinforcing cycle (the ‘vicious cycle’) that perpetuates the inflammation and ocular surface damage in DED.4-64
“ The aim of treatment for DED is to restore homeostasis of the ocular surface, by breaking the vicious inflammatory cycle of the disease and preventing its return ”
RISK FACTORS
Factors that increase evaporative loss of the tear film (e.g., high wind speeds, low humidity) or reduce lacrimal gland tear secretion (e.g., sensory nerve damage from refractive eye surgery, hormonal changes), certain medications (e.g., β-blockers, diuretics, antihistamines), and some systemic diseases (e.g., Sjögren’s syndrome) contribute to the pathophysiological process occurring at the ocular surface and increase the risk of developing DED. 6
Factors that act at the ocular surface to initiate tear film instability (e.g., ocular allergy, topical preservative use, contact lens wear) also increase the risk of DED.66Additionally, exposure to air pollution and tobacco smoke may contribute to the inflammatory response at the ocular surface.
TREATMENT RECOMMENDATIONS
The aim of treatment for DED is to restore homeostasis of the ocular surface, by breaking the vicious inflammatory cycle of the disease and preventing its return. 8
The Tear Film and Ocular Surface Society's Dry Eye Workshop II (TFOS DEWS II) Management and Therapy Report has a flexible four-step approach to treatment of DED, based on disease aetiology and severity. It recommends anti-inflammatory therapy, i.e., topical corticosteroids and ciclosporin, as a step two add-on therapy if step one options (e.g., education, lifestyle changes, use of preservative-free artificial tears [PF-ATs], and eye lid therapy) prove to be inadequate for treating DED.8
Although PF-ATs, which are the mainstay of initial treatment for DED,88can help to ameliorate ocular surface damage by reducing friction or alleviating tear hyperosmolarity, they have relatively short persistence and do not address the underlying inflammatory pathophysiology of DED. 4,5
Topical corticosteroids are effective in breaking the cycle of ocular surface inflammation and damage in DED and are an established therapeutic intervention for DED.8,9 However, the duration of use of topical corticosteroids is limited due to potential adverse events (AEs), including ocular hypertension, cataracts, and opportunistic infections, even with shortterm use. Nonetheless, short-term use may be beneficial when initiating ciclosporin. Pre-treatment with a short course of topical corticosteroid has been demonstrated in clinical studies to provide more rapid improvement in symptoms as well as in clinical signs than with ciclosporin alone, and to reduce the frequency of severe stinging related to topical ciclosporin. 8
Comparative clinical trials and meta-analyses support the efficacy of topical ciclosporin in the treatment of DED, with an acceptable safety profile.8,9 Moreover, unlike PF-ATs, ciclosporin targets the inflammatory process of DED and, unlike corticosteroids, ciclosporin can be safely administered for extended periods.
Although ciclosporin therapy is typically started when there is substantial corneal and/or conjunctival staining, the early initiation of ciclosporin should be considered in patients who present with risk factors for severe DED, such as corneal and conjunctival staining, inadequate control with standard-of-care treatment (e.g., artificial tears), rapid response to corticosteroid therapy, or presence of immune-related systemic disease.9
Risk factor identification can facilitate early diagnosis of DED and early diagnosis is beneficial to treatment and a more favourable prognosis.10,11
Ciclosporin therapy may be continued indefinitely if it remains well tolerated and effective; however, tapering and discontinuation of ciclosporin, with ongoing monitoring for disease recurrence, should be considered if a sufficient and sustained improvement is achieved.9,12
Ciclosporin Mechanism of Action
Ciclosporin is an immune-modulating cyclic polypeptide with anti-inflammatory properties. 8,13 When administered topically, ciclosporin is thought to act primarily by inhibiting that part of the immune system that relies on proliferation of T cell lymphocytes.
As an inhibitor of the T cell-activator calcineurin, ciclosporin prevents the activation of transcription factors required for the activation of T cells and the subsequent release of pro-inflammatory cytokines by activated T cells.13,14 Hence, ciclosporin is able to disrupt immune-mediated inflammatory responses at the ocular surface. Ciclosporin has also been demonstrated to increase goblet cell density and block the pathological apoptosis of secretory conjunctival epithelial cells, 15,16 which may improve the health of the ocular surface and tear film quality.
Ciclosporin Efficacy
In clinical and real-world studies, topical ciclosporin demonstrated improvements in symptoms and clinical signs in patients with DED, which may be evident within four weeks of starting treatment. 9,12
Studies have also demonstrated that long term use of topical ciclosporin can provide ongoing benefits to patients with DED and the potential for symptoms and clinical signs to resolve during longer-term treatment.9 Absence of symptoms and clinical signs for up to two years after responding to an initial course of treatment with ciclosporin has been observed in some DED patients.17,18
Tolerability
Ciclosporin administered enterally or parenterally is associated with systemic AEs including renal impairment, potentially leading to hypertension, lymphoma, and gingivitis. 19,2019,20However, topical administration of ciclosporin results in little or no systemic drug absorption, and accordingly has a lower risk of systemic AEs. 21,22
Most treatment-emergent adverse events (TEAEs) reported in clinical and real-world studies of topical ciclosporin were ocular in nature, with the most common TEAEs being ‘stinging’ and irritation.9 TEAEs were mainly mild or moderate in severity in clinical studies and only a small proportion of patients discontinued use of topical ciclosporin because of TEAEs. There is also evidence of a reduction in ocular TEAEs with ongoing administration of ciclosporin. 12
Favourable longer-term tolerability of topical ciclosporin has been demonstrated in clinical studies of up to 36 months’ duration, with no specific safety concerns identified.9,12 The frequency of systemic AEs related to longer-term treatment with topical ciclosporin was very low and serious systemic effects were not reported.
PATIENT MANAGEMENT
Eye care practitioner education of patients with DED about their condition and moderation of their expectations for treatment are critical to maximise patient adherence to ciclosporin therapy and minimise treatment discontinuation (Table 1). 9,12
Ciclosporin ophthalmic solutions should not be administered into eyes wearing contact lenses because the vehicle tends to adhere to the lens and form deposits.12 Contact lenses should be removed prior to instillation of ciclosporin ophthalmic solution and may be reinserted 15 minutes after instillation.
Before starting treatment, patients should be advised of the possibility of instillationrelated AEs with ciclosporin therapy, such as ocular burning, stinging, and redness.9,12 If these AEs are encountered, patients should be encouraged to continue treatment since they usually resolve over time as the health of the ocular surface improves.12
An additional step that patients can take to mitigate instillation-related ocular AEs is to use PF-ATs 15–20 minutes before and/or after ciclosporin.9,12 Additionally, the temporary use of topical corticosteroids one to three weeks prior or concurrent with ciclosporin therapy may help to reduce instillation-related ocular AEs and promote a more rapid improvement in symptoms and clinical signs of DED.
Patients should also be informed that long-term therapy with ciclosporin may be required in chronic cases and in most patients indefinite treatment is necessary to prevent relapse.9,12
Counselling patients to not expect immediate symptom improvement after starting ciclosporin will help to facilitate adherence to ciclosporin therapy.9,12 The delay in symptom relief with ciclosporin, which could be up to three months after starting therapy, may be due to its mechanism of action targeting non-activated T cells rather than already-activated T cells. 12
TREATMENT FOR MODERATE-TO-SEVERE DRY EYE DISEASE
Anti-inflammatory therapy is required in patients with moderate-to-severe DED to break the cycle of ocular surface damage and inflammation.23
In Australia, ciclosporin 0.09% (Cequa), as eye drops for topical ophthalmic use, is indicated to increase tear production in patients with moderate-to-severe DED where previous use of artificial tears has not been sufficient (Table 2).24
“ … the duration of use of topical corticosteroids is limited due to potential adverse events (AEs), including ocular hypertension, cataracts, and opportunistic infections, even with short-term use ”
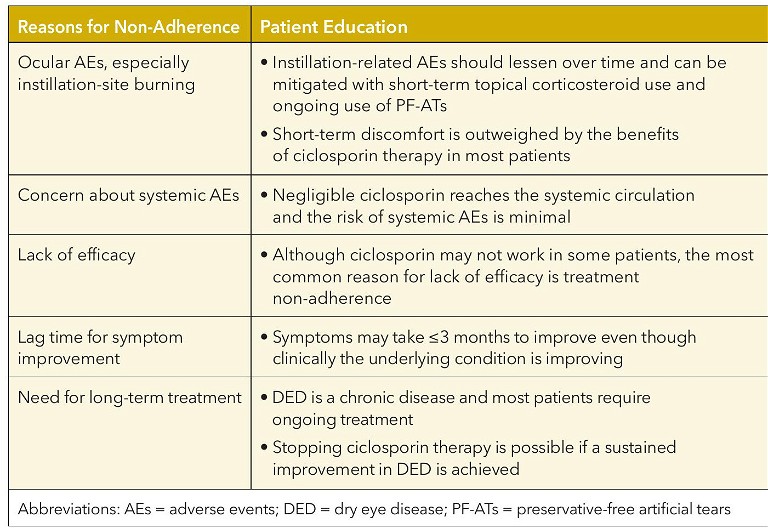
Table 1. Potential reasons for non-adherence to ciclosporin therapy and patient education to optimise adherence to therapy.9,12
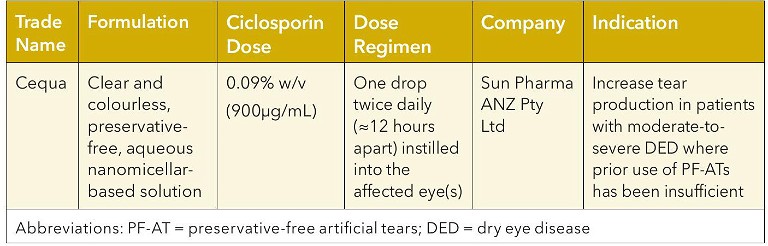
Table 2. Topical ciclosporin formulation for treatment of moderate-to-severe DED.24
CICLOSPORIN 0.09%
The hydrophilic stroma of corneal and conjunctival tissues impedes the permeation of hydrophobic molecules. 25 As a hydrophobic molecule, ciclosporin has poor aqueous solubility and hence low ocular tissue bioavailability. 26 To allow for improved drug solubility and hence penetration of ciclosporin into corneal and conjunctival tissues, ciclosporin 0.09% is formulated in a micellar nanosized carrier system. 22
Nanomicelles are nanosized vesicular carriers that possess both hydrophilic and hydrophobic groups, a characteristic that allows nanomicelles to encapsulate hydrophobic ciclosporin and facilitate its ocular delivery via aqueous channels.2525The hydrophilic nature of the nanomicellar carrier is also thought to minimise wash out of ciclosporin into the systemic circulation via conjunctival/choroidal blood circulation and lymphatics.
Extensive penetration of ciclosporin into corneal and conjunctival tissues, with minimal systemic drug accumulation, following repeated ocular instillation of nanomicellar ciclosporin 0.09% has been demonstrated in a preclinical study.27 In addition, systemic exposure to ciclosporin was demonstrated to be negligible in human volunteers after repeated ocular administration of nanomicellar ciclosporin 0.09% in a Phase 1 clinical study. 28 Negligible systemic exposure to ciclosporin suggests a lower risk for systemic drug-related AEs. 22
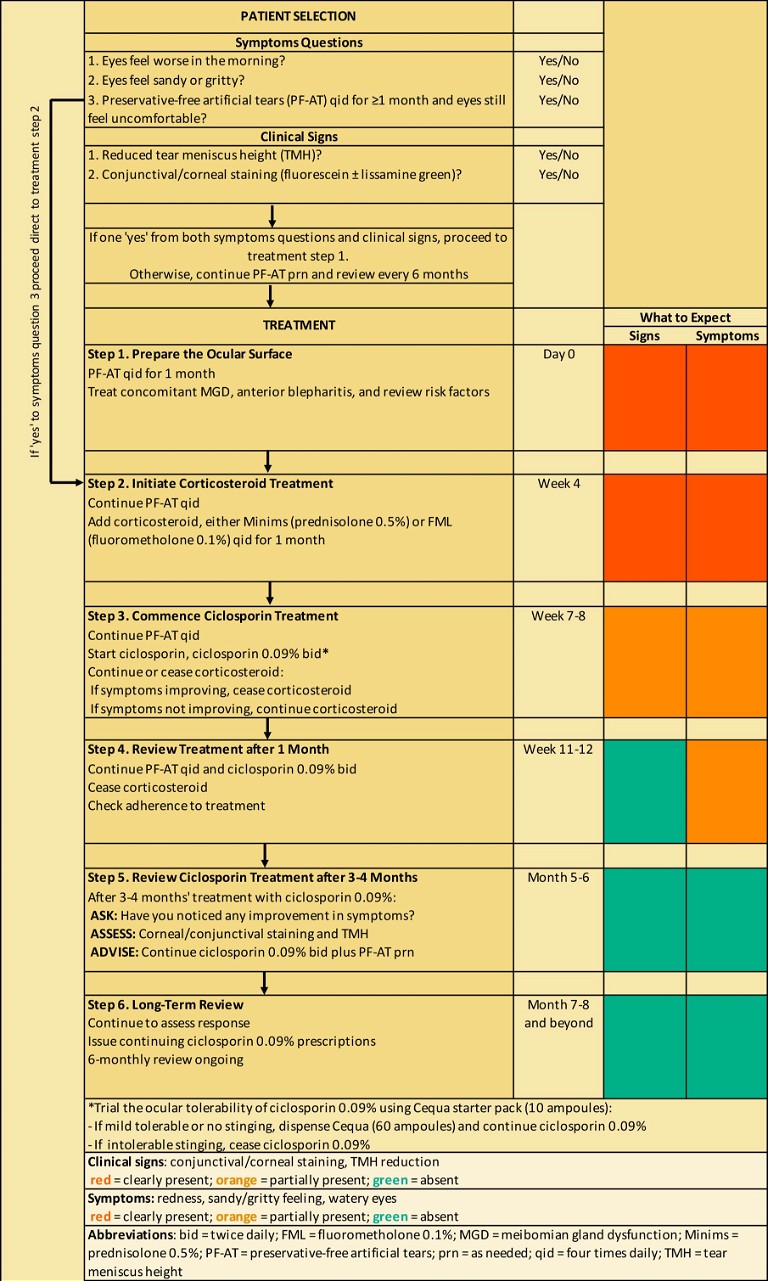
Figure 1. Algorithm for anti-inflammatory treatment of patients with moderate-to-severe DED.
Efficacy and Tolerability
Three pooled analyses of data from two pivotal clinical trials (one Phase 2/3 and one Phase 3 trial) have demonstrated that ciclosporin 0.09% was more effective than vehicle in reducing corneal damage and ocular surface inflammation and improving tear secretion in patients with moderateto-severe DED, hence suggesting improved ocular surface health. 29-31
The pooled data also demonstrated that ciclosporin 0.09% was generally well tolerated, with most ocular TEAEs being of mild-to-moderate severity.29-31 Additionally, data from a one-year extension study of the Phase 3 pivotal clinical trial demonstrated that ciclosporin 0.09% was safe and generally well tolerated in patients with DED,32 hence providing support for longer-term use of ciclosporin 0.09%. Temporary mild stinging or burning after instillation was the most commonly reported TEAE, occurring in approximately 22% of patients who received ciclosporin 0.09% and approximately 4% of patients who received vehicle. 29,31
PRESCRIBING CONSIDERATIONS
Cequa starter packs (containing 10 ampoules for single use) are available for eye care practitioners to give to patients when writing prescriptions for ciclosporin 0.09%. Starter packs allow patients to trial ciclosporin 0.09% for ocular tolerability. Approximately 2.4% of patients discontinued ciclosporin 0.09% due to ocular AEs in a Phase 3 clinical trial.33
Patients experiencing mild tolerable stinging or no stinging while on the Cequa starter pack should be advised to dispense and obtain Cequa from the pharmacy and continue ciclosporin 0.09% therapy. Patients who experience intolerable stinging while on the trial should discontinue use.
If patients do continue beyond the starter pack, it is important to instruct them not to stop using ciclosporin 0.09% during the first three to four months of use, even if there is no improvement in symptoms. Data from the series of clinical trials showed significantly improved corneal and conjunctival staining scores were evident as early as 28 days after starting ciclosporin 0.09%, whereas symptomatic improvement was not observed until day 84 after starting treatment.29,30,33
When using ciclosporin 0.09% concomitantly with PF-ATs, allow a 15-minute interval between instillations.24
Treatment Algorithm
An easy-to-follow algorithm has been developed to assist eye care practitioners with determining when anti-inflammatory therapy is appropriate and how to initiate and manage anti-inflammatory treatment with ciclosporin 0.09% in patients with moderateto-severe DED (Figure 1). A combination of symptoms triage and clinical signs assessment using non-invasive tests is recommended to determine whether a DED patient requires anti-inflammatory treatment.
Symptoms triage comprises three simple (yes or no) questions to assess the degree of eye discomfort due to DED and whether use of PF-ATs has been beneficial.
Corneal staining, conjunctival staining, and TMH measurement are clinical tests that are commonly employed in eye care clinics. Reduced TMH indicates a reduction in tear film volume, and hence sub-optimal ocular surface health. Ocular staining tests determine if ocular surface damage is present (yes or no): fluorescein staining detects corneal damage (a positive result is >5 corneal spots) and lissamine green staining detects conjunctival damage (a positive result is >9 conjunctival spots).
At least one positive (‘yes’) result in each of the symptoms triage and clinical signs assessment sections indicates the need for anti-inflammatory therapy starting at treatment step one (Day 0; preparing the ocular surface with PF-ATs and treatment of secondary conditions) except when there is a positive answer to symptom triage question number three indicating inadequacy of intensive PF-ATs alone, in which case starting at treatment step two is recommended.
“ An easy-to-follow algorithm has been developed to assist eye care practitioners with determining when antiinflammatory therapy is appropriate ”
Treatment step two (i.e., after four weeks of PF-ATs use) involves starting a short-term corticosteroid, either minims (prednisolone 0.5%) or FML (fluorometholone 0.1%), with continued use of PF-ATs. The addition of a topical corticosteroid also helps to prepare the ocular surface by reducing ocular inflammation. Use of a corticosteroid also ameliorates any ocular discomfort associated with ciclosporin and may contribute to more rapid symptom improvement.8,9 Additionally, a positive response to corticosteroids may indicate likely treatment response to ciclosporin.
Anti-inflammatory therapy with ciclosporin 0.09% is initiated at treatment step three (i.e., after three to four weeks of corticosteroid use) with or without concurrent adjuvant corticosteroid use (depending on any symptom improvement with corticosteroid use) and continued use of PF-AT. Note that continuation of ciclosporin 0.09% therapy assumes a successful trial of its ocular tolerability using the Cequa starter pack.
At treatment step four (i.e., after four weeks of ciclosporin therapy), ongoing corticosteroid use is discontinued, ciclosporin and PF-ATs are continued, signs and symptoms may be reviewed and patient adherence with treatment is checked.
At treatment step five (i.e., after three to four months of ciclosporin 0.09% therapy), symptoms and clinical signs are re-assessed and if results for both indicate treatment response, the continuation of ciclosporin plus PF-ATs is recommended.
Treatment step six, long-term review, involves issuing continuing ciclosporin 0.09% prescriptions and assessment of response every six months.
In the treatment algorithm (Figure 1), a traffic light system is used to visually indicate the expected response at different treatment duration time points in terms of symptoms and clinical signs (green = absent; orange = partially present; red = clearly present). The colour graduations of the traffic light system serves to remind eye care practitioners that an improvement in symptoms with ciclosporin therapy typically lags improvements in clinical signs.
CONCLUSION
Ocular surface inflammation and damage, driven by activation of T cells that release inflammatory mediators, play a key role in the pathophysiology of DED. Ciclosporin has been demonstrated to prevent T cell activation, which helps to break the inflammatory cycle of DED and increase lacrimal gland tear secretion in DED patients.
The use of topical ciclosporin is recommended as an anti-inflammatory therapy for DED. Ciclosporin 0.09% is indicated for the treatment of moderate-to-severe DED that has not responded adequately to treatment with PF-ATs. Consideration of initiating ciclosporin therapy earlier in the disease course has been proposed. Preparation of the ocular surface with PF-ATs and short-term corticosteroids will minimise the ocular AEs associated with ciclosporin therapy. Critical to the success of anti-inflammatory therapy with ciclosporin is patient education; in particular, advising patients of a delay in the onset of symptomatic improvement and that long-term treatment is usually necessary.
Adherence to long-term ciclosporin therapy will likely be rewarded with improved treatment outcomes in most DED patients and may delay or halt disease progression in some patients.
To earn your CPD hours from this article visit mieducation.com/treating-dry-eye-with-ciclosporin0.09%-practical-guidance-for-eye-care-practitioners.
This article was commissioned by Sun Pharma, manufacturer of Cequa.
The brand names Cequa, Minims, and FML are registered trademarks to their relevant manufacturers.
References available at mieducation.com

Jason Holland B.App.Sci (Hons) (Optom) PGOT CASA CO runs a glaucoma and advanced dry eye clinic in Brisbane. He is the national director of optometry for the Optical Superstore Group, and President of the Dry Eye Society.

Dr Margaret Lam B.Optom GradCertOcTher is the National President for Optometry Australia and the National Vice President of the Cornea and Contact Lens Society of Australia. She is the head optometrist at 1001 Optical in Bondi Junction Sydney and teaches at the School of Optometry and Vision Science at the University of New South Wales.

Dr Leigh Plowman B.Optom (Therapeutics) is an optometrist at Otway Optical in Colac, Victoria. He is the founder of the Dry Eye Directory and has written and presented on dry eye disease.

Hugh Bradshaw B.App.Sc (Optom) GradCertOcTher CASA CO is an optometrist and director of Heron Eyecare in Toowoomba, Queensland. He has a special interest in dry eye disease treatment and eye disease diagnosis and management. Mr Bradshaw is also an education committee joint chair of the Dry Eye Society.

Jason Teh BSc (Hons), B.Optom is an optometrist and the founder and director of the Dry Eye Group with three practices across Melbourne specialising in the assessment, treatment and management of dry eye disease. Mr Teh runs dry eye workshops and lectures on DED at various conferences.

Daniel Tracton B.Optom (Hons) GradCertOcTher MBA is an optometrist and the owner of Tracton Optometrists in Bondi Junction in Sydney. He has a keen interest in dry eye.