eyecare
Hydrus Microstent
Milestones at 2 and 5 Years
WRITERS Dr I Paul Singh, Dr Brian Flowers, Professor Pradeep Ramulu
Alcon’s Hydrus Microstent is a minimally invasive surgical device that received regulatory approval on the basis of findings from the two-year HORIZON trial. Recently published results of five years of follow-up have confirmed safety and efficacy outcomes.
The HORIZON trial, a Phase 3 clinical trial that compared Hydrus Microstent (Alcon) plus cataract surgery (CS) to CS alone, was the largest minimally invasive glaucoma surgery (MIGS) pivotal trial conducted to date, with 38 sites in nine countries.1-3
Approximately 40% of the Hydrus patient population came from outside the United States. For the study, patients with mild-to-moderate primary open-angle glaucoma (POAG) on one to four medications underwent CS and then were randomised 2:1 to Hydrus Microstent placement (n=369) or CS alone (n=187).1
The primary endpoint of the study was the percentage of patients with a 20% or greater reduction in washed-out diurnal intraocular pressure (DIOP) after two years of follow-up. The main secondary endpoint was the change in washed-out DIOP at two years.1 In the second phase of the study, patients were continuously followed for an additional three years to monitor safety outcomes and to assess predefined efficacy endpoints.2,3 Notably, approximately 80% of patients (n=442/556) originally enrolled to receive Hydrus Microstent were followed to the fiveyear study endpoint.3
THE PIVOTAL PHASE: YEAR TWO DATA
The study met its primary endpoint at two years (Figure 1).1 The mean reduction in washed-out DIOP at two years was -7.6 ±4.1 mm Hg and -5.3 ±3.9 mm Hg in the Hydrus plus CS group and CS alone group, respectively (difference = -2.3 mm Hg; P <.001). The mean number of medications was reduced from 1.7 ±0.9 to 0.3 ±0.8 in the Hydrus plus CS group and from 1.7 ±0.9 to 0.7 ±0.9 in the CS alone group (difference = -0.4 medications; P <.001).1
As for safety, there was a low percentage of adverse events overall. Postoperative cell and flare were more common in the Hydrus plus CS group in the first week, but all cases resolved within the first month.1 The most common adverse event reported in the Hydrus plus CS group was peripheral anterior synechiae (PAS) or iris tissue near the inlet; these were considered obstructive in 14 of 369 (3.8%) affected eyes and nonobstructive in 55 of 369 (14.9%) eyes. The investigators noted that observation of PAS or adhesion did not affect the outcome.1
EXTENDED FOLLOW-UP: YEAR FIVE DATA
The percentage of subjects with reported serious adverse events was 3.5% in the Hydrus plus CS group (n=13/369) and 4.3% in the CS alone group (n=8/187).3 There was no difference from year two to five in secondary safety outcomes, except for the rate of PAS, which was significantly higher at five years in the Hydrus plus CS group (14.6%) compared to CS alone (3.7%; P=.0001). However, the majority of eyes with PAS in the Hydrus Microstent group (8.7%) were not device obstructing, and there was no difference in intraocular pressure (IOP) between patients with (16.9 ±3.3 mm Hg) and without PAS (16.6 ±3.5 mm Hg; P=.49). In the majority of eyes with PAS (30/42; 71.4%), PAS were focal or less than one clock hour in size. There was no impact on IOP or visual acuity due to PAS, and there were no sequelae observed in eyes with device obstruction.3
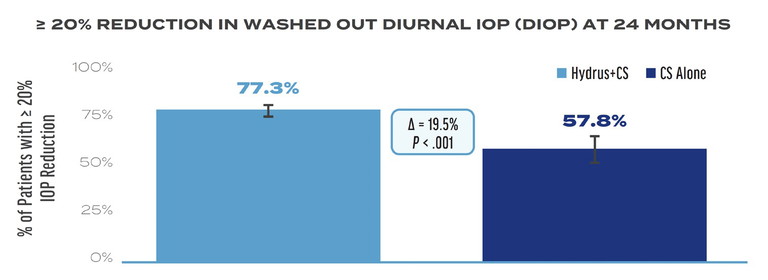
Figure 1. A greater percentage of patients achieved ≥20% reduction in washed-out diurnal IOP at 24 months in the Hydrus Microstent plus cataract surgery (CS) group compared to CS alone, which was the primary endpoint in the HORIZON trial.1
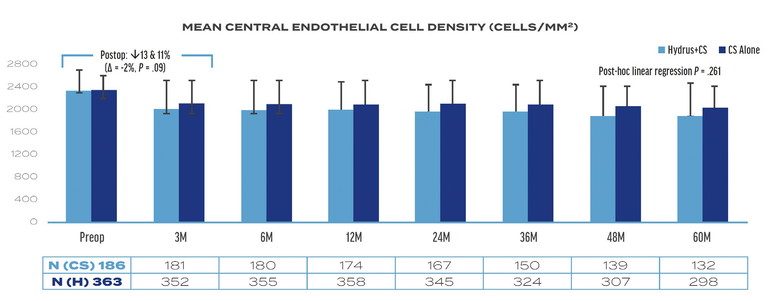
Figure 2. Mean central endothelial cell density in the Hydrus plus cataract surgery and CS alone groups at five years in the HORIZON trial.3
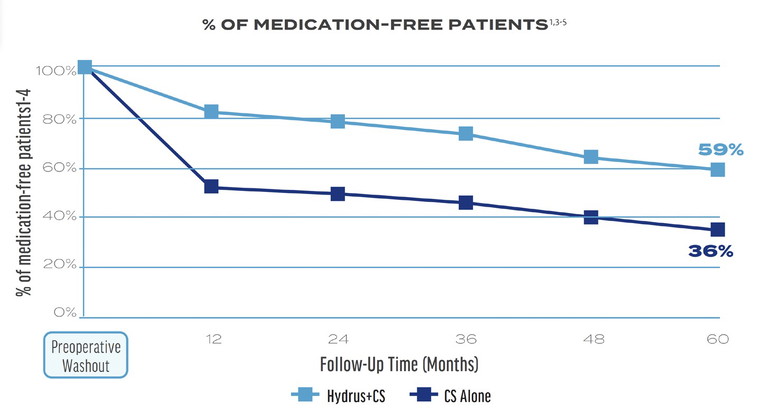
Figure 3. A higher percentage of patients in the Hydrus Microstent plus CS group achieved medication-free status at five years compared to the CS alone group.**
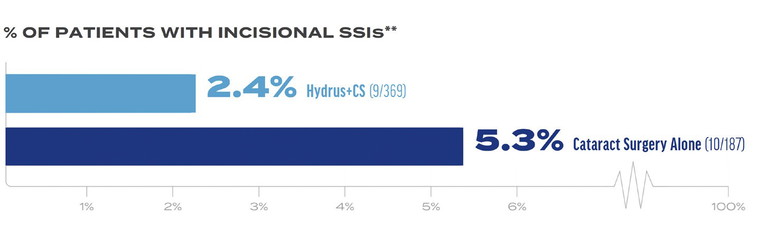
Figure 4. Hydrus Microstent plus CS delivered a 50% reduction in incisional secondary surgical interventions (SSIs) compared to CS alone.3 **
The investigators observed a 2% between-group difference in mean central endothelial cell density (ECD) at three months postoperatively (11% rate of endothelial cell loss [ECL] CS alone vs 13% ECL Hydrus plus CS; P=.08). The difference may be attributable to the additional manipulation required when inserting the Hydrus Microstent. The between-group difference increased to 6% over five years (13% ECL CS alone vs 19% ECL Hydrus plus CS), but the difference was not statistically significant (Figure 2).3
In addition to ECD values alone, the rate of change of ECD was looked at. In the study, the year-to-year ECL ranged from zero to 2% in the Hydrus plus CS group and zero to 1% in the CS alone group. There was no statistically significant difference in the rate of ECL from three months to five years in either group, and there was no shift toward increased ECL in years four or five compared to prior years.
Of note, at the five-year follow-up, the proportion of eyes with ≥30% ECL increased from 17.3% at three months to 20.8% (P=.27) in the Hydrus plus CS group and from 9.4% at three months to 10.6% (P= .85) in the CS alone group. Logistic regression showed no difference in the rate of change of ≥30% ECL between the Hydrus plus CS group compared to the CS alone group from three months to five years (P=.82). Furthermore, no eyes with ≥30% ECL in the Hydrus plus CS or CS alone groups had associated clinical sequelae.3
As for secondary effectiveness outcomes, a higher percentage of patients achieved medication-free status with Hydrus plus CS versus CS alone (Figure 3),3 and Hydrus plus CS was associated with a reduction in the rate of secondary incisional surgery (Figure 4).3
Dr I Paul Singh is a glaucoma specialist at the Eye Centres of Racine and Kenosha, in Wisconsin, USA. He is a member of the Glaucoma Today Editorial Advisory Board.
Dr Brian Flowers is a glaucoma specialist and the managing partner of Ophthalmology Associates in Fort Worth, Texas, USA.
Professor Pradeep Ramulu is the chief of the glaucoma division, and Professor of Ophthalmology, at Johns Hopkins Wilmer Eye Institute, Baltimore, USA.
** SSIs include trabeculectomy, tube shunt, gel stent, ECP/ TSCP, non-penetrating; (9/369 Hydrus and 10/187 CS).
References
1. Samuelson TW, Chang DF, Singh K, et al. HORIZON Investigators. A schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: The HORIZON study. Ophthalmology. 2019 Jan;126(1):29-37. doi: 10.1016/j.ophtha.2018.05.012.
2. Ahmed IIK, Rhee DJ, Singh K, et al. HORIZON Investigators. Three-year findings of the HORIZON trial: A schlemm canal microstent for pressure reduction in primary open-angle glaucoma and cataract. Ophthalmology. 2021 Jun;128(6):857-865. doi: 10.1016/j.ophtha.2020.11.004.
3. Ahmed IIK, De Francesco T, Singh K, et al. HORIZON investigators. Long-term outcomes from the HORIZON randomized trial for a schlemm's canal microstent in combination cataract and glaucoma surgery. Ophthalmology. 2022 Jul;129(7):742-751. doi: 10.1016/j.ophtha.2022.02.021.
4. Alcon Data on File, 2024.
5. Hydrus microstent [instructions for use]. Irvine, CA: Alcon Vision LLC; September 2021 (United States).