education
mieducation
More Than Meets the Eye
Pseudoexfoliation Syndrome
Pseudoexfoliation syndrome (PXF) is the most common identifiable cause of open-angle glaucoma, with PXF-affected individuals demonstrating an approximately 10 times risk of developing glaucoma.1 Even more clinically concerning for those affected individuals, pseudoexfoliative glaucoma (PXG) is one of the most aggressive and clinically complex forms of secondary open angle glaucoma. This type of glaucoma is associated with higher intraocular pressure (IOP) levels, greater IOP fluctuation, more advanced optic nerve damage at diagnosis, as well as a faster rate of progression when compared to primary open angle glaucoma (POAG).2
Nicole Lawson provides a primer on pseudoexfoliation syndrome (PXF) and pseudoexfoliative glaucoma (PXG), concluding that the key to appropriate patient care is recognising PXF as a multisystem disorder.
WRITER Nicole Lawson
LEARNING OBJECTIVES
On completion of this CPD activity, participants should:
1. Recognise pseudoexfoliation (PXF) syndrome as a multifactorial disorder,
2. Be aware of the epidemiology and incidence of PXF,
3. Understand the pathophysiology, risk factors and clinical implications of PXF, and
4. Be aware of current and emerging treatment strategies.
Pseudoexfoliation syndrome is characterised by the accumulation of fibrillar extracellular material within ocular tissues,3 with the increased glaucoma risk predominately due to the accumulation of insoluble PXF material in ocular outflow structures, leading to increased outflow resistance and elevated IOP. Although PXF often presents unilaterally in its early stages, it progresses to being clinically evident bilaterally in up to 50% of cases within 15 years.4 Notably, histopathological studies have demonstrated subclinical involvement of the fellow eye, even when PXF material is not yet detectable on clinical examination.5
Identification of similar extracellular material accumulation on other organs in affected individuals has led to a growing acceptance that PXF is actually a systemic condition. These potential systemic comorbidities and the aggressive nature of associated glaucoma progression mean that early diagnosis and prompt initiation of treatment are critical in altering the disease trajectory and preserving vision.
EPIDEMIOLOGY
The epidemiology and incidence of PXF varies markedly with geographic location and ethnicity, with the underlying factors still not being entirely understood. It is also important to note that subclinical and early stages of PXF may be missed on clinical examination, and thus true prevalence may not be known.
Where Does Pseudoexfoliative Material Come From?
The exact locations from where PXF material arises are still not well understood. It is hypothesised to be synthesised from the iris, lens epithelium, ciliary body or trabecular meshwork.14
Immunostaining and differential proteomic analysis have been used to better understand the components of PXF material, providing insight into potential pathophysiology.15 Anterior lens capsules of patients with PXF that were removed during routine cataract surgery have been analysed using these methods and compared to healthy control patients.15
The components of PXF material can be separated into three different components: basement membrane, elastic fibre, and blood-derived.16 The presence of basement membrane components suggests that aberrant basement membrane synthesis and turnover contribute to extracellular matrix dysregulation and formation of PXF material. The presence of elastic fibre components suggests a degenerative elastotic process, which involves abnormal breakdown or changes in elastic tissues. The presence of multiple complement factor proteins suggests that the degenerative elastotic process may promote the recruitment of inflammatory mediators, complement components, and other serum-derived proteins into the aqueous humour. This implies that an underlying inflammatory response, in conjunction with compromised integrity of the blood–aqueous barrier, may facilitate the abnormal leak of serum proteins into the anterior chamber, resulting in the development of PXF material.
The prevalence rates increase with age and can be as high as 20–30% in those aged over 70 years old in some regions, in particular Scandinavia and Greece.6 In contrast, PXF was considered rare in Chinese people, with early studies reporting very low prevalence rates, such as 0.2% in the Singaporean Tanjong Pagar Study.7 However, the Beijing Eye Study reported a much higher overall prevalence of 5.8% among Chinese subjects, indicating that the condition may not be as rare as previously thought in this population.8
In the United States and Israel, there is a positive association observed between residential distance from the equator and PXF, suggesting that ocular exposure to light from reflective surfaces, such as snow or water, may be important for PXF development.9 However, this does not fully explain the variability, as some populations with significant exposure to reflected sunlight, like Inuits from Greenland, have a relatively low prevalence of PXF.10
In Australia, the prevalence of PXF syndrome has been reported as 1–4.7%, depending on the study, however these epidemiology studies were performed over 10 years ago and may not be representative of today’s population.11-13 PXF also has a higher prevalence among Indigenous Australians, with up to 16% in individuals aged over 60, compared to approximately 3% in non-Indigenous Australians in the same age group.13
PATHOPHYSIOLOGY AND RISK FACTORS
Despite extensive research, the precise cellular and molecular mechanisms underlying the production of pseudoexfoliative material remain incompletely understood, with the underlying pathophysiology being multifactorial (Figure 1).17
As mentioned previously, degenerative elastotic processes and underlying inflammation may play a role. Oxidative stress may also contribute to the formation of PXF material, with the eye being particularly susceptible due to its high metabolic activity and increased exposure to light.18 Oxidative stress may impair cellular processes, leading to formation of PXF material.
Genetic factors have also been implicated in the pathogenesis of PXF. Genetic variants in the lysyl oxidase-like 1 (LOXL1) gene were the first major genetic risk factor identified to be associated with the development of PXF19 with others emerging since.20 The disease also tends to cluster within families, however genetics alone do not account for the development of PXF, as individuals with the specific identified genetic variants may not develop the condition, highlighting the multifactorial nature of the disease.
Additionally, epigenetics, which influence how genes are expressed, may also play a role.20
Age is the most notable risk factor for PXF, with prevalence increasing with age.20 Ageing also correlates with diminished tissue elasticity and impaired cellular repair mechanisms. Bilateral manifestation tends to present later and at a more severe stage compared to the unilateral presentation, possibly implicating the role of ageing and disease trajectory.20 Additionally, increasing age may result in accumulation of other risk factors, such as environmental exposure, as prolonged exposure to ultraviolet radiation has been linked to increased disease risk.21
Dietary and lifestyle influences, such as high caffeine intake, smoking, alcohol consumption, and reduced folate or vitamin B12, have also been implicated.21 Previous intraocular surgery may also disrupt the blood-aqueous barrier, potentially facilitating protein leakage and development of PXF material.22 Systemic conditions such as cardiovascular disease have been linked to PXF, however the causality and directionality of these associations remain unclear.23
CLINICAL FINDINGS AND IMPLICATIONS
In 1917, a Finnish ophthalmologist, Dr John Lindberg, first described greyish flecks on the pupillary border associated with chronic glaucoma.24 The finding was then later termed ‘pseudoexfoliation’ by Dr Georgiana Dvorak-Theobald in 1954 to distinguish the disease from true exfoliation syndrome, a rare condition involving delamination of the anterior lens capsule.25 However, the condition may be referred to as both pseudoexfoliation and exfoliation syndrome in the literature.
In a clinical exam, PXF material is typically observed by slit-lamp examination along the pupil margin and on the anterior lens (Figures 2A and 2B). Although less common, the material may deposit on the corneal endothelium and may resemble Fuchs’ dystrophy. Individuals with PXF have increased rates of reduced endothelial cell density, as well as endothelial polymegathism and pleomorphism.26 Pigment deposition on the corneal endothelium may also be present, likely due to the release of pigment from the iris pigmented epithelium abrading against the material on the anterior lens. There may be loss of the pupillary ruff, however this sign is not diagnostic and may be observed in individuals without PXF.
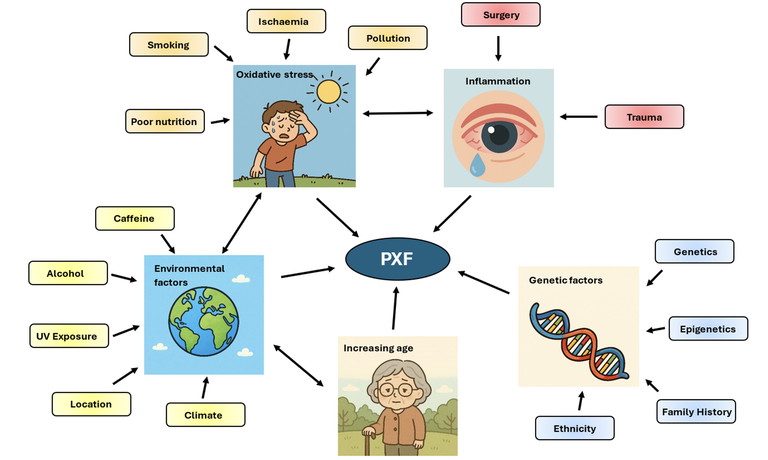
Figure 1. Factors associated with the development of pseudoexfoliative material adapted from Mastronikolis et al.17
Gonioscopy is essential in PXF (Figure 3), with the angle appearance tending to be narrower than the general population, likely secondary to weakened zonules causing anterior lens displacement.27 There tends to be increased pigmentation of the trabecular meshwork with a patchier and mottled appearance compared to the homogeneous distribution observed in pigment dispersion syndrome. Pigment may also deposit along Schwalbe’s line with Sampolesi’s line being observed, however this is not universal. PXF material may be visible within the angle, exhibiting a flaky, dandruff-like appearance, and posterior synechiae may also be present.
Poor pharmacological mydriasis is a common feature of PXF, complicating both clinical evaluation and intraocular surgery. This is thought to result from deposition of material within the iris stroma and dilator muscle, leading to muscle fibre degeneration and reduced tissue elasticity.28 Additionally, vascular dysregulation and decreased iris perfusion may contribute to impaired dilation. Along with zonular weakness, this poses a particular challenge for cataract surgery, necessitating an earlier referral for surgical intervention. There is an increased risk of lens subluxation or dislocation, with phacodonesis and/or lens subluxation being reported in up to 10% of cases.29 Capsular contraction is also more frequent, prompting the use of a larger capsulorhexes, capsular tension rings, and the consideration of intraocular lenses with reinforced haptics for improved postoperative stability.30 It is important to note that cataract surgery does not cure the condition, but may provide a reduction in IOP.31
Dry eyes are associated with PXF; individuals tend to have lower basal tear test scores, decreased tear break-up times, loss of goblet cell density, and meibomian gland dysfunction.1 This has implications for potential topical management.
SYSTEMIC IMPLICATIONS
PXF is increasingly recognised as a systemic disorder characterised by the extracellular accumulation of fibrillar material, not only within ocular structures, but also in multiple extraocular tissues, including the heart, lungs, kidneys, liver, gallbladder, and brain.32 The deposition of pseudoexfoliative material in vascular tissues is thought to contribute to increased vascular resistance, impaired perfusion, arterial dysregulation, and autonomic vascular dysfunction.
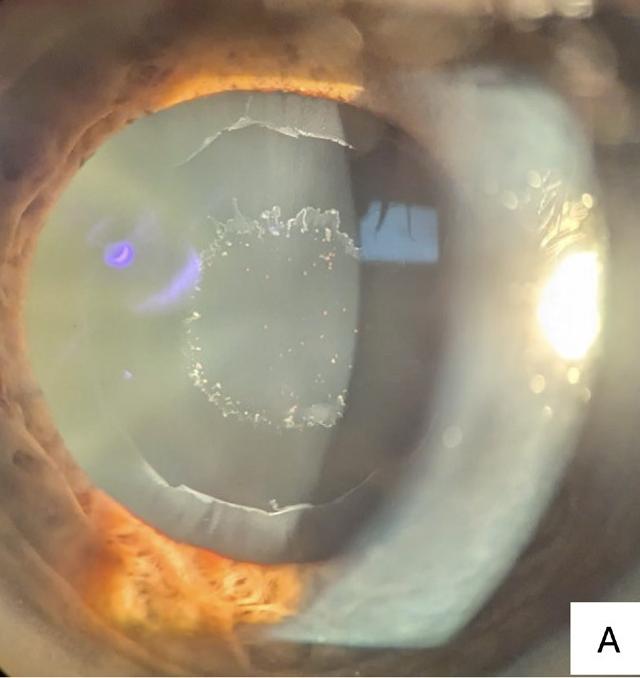
Figure 2. (A) Pseudoexfoliative material deposited on the anterior lens of an 80-year-old patient who attended the Centre for Eye Health (CFEH). There are three distinct zones with the inner ring typically corresponding with the pupil size, the clear intermediate zone, and the outer ring, often observed with a jagged inner edge.
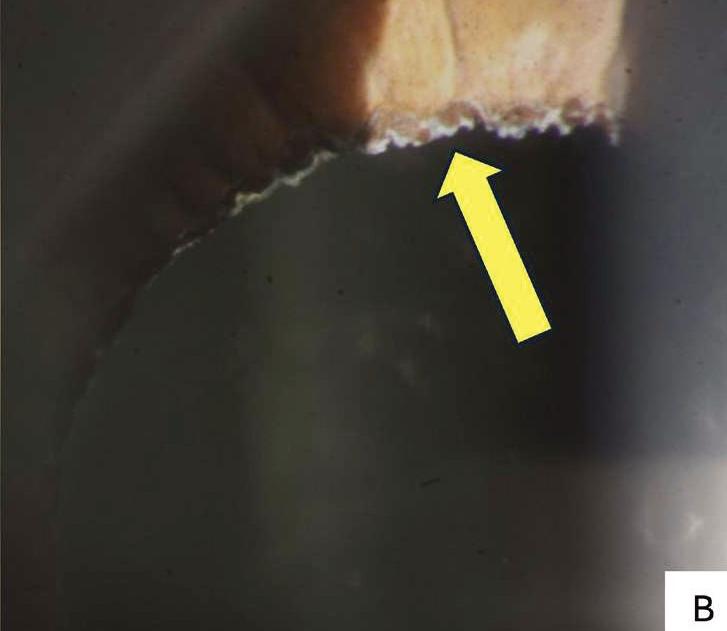
(B) Pseudoexfoliative material deposited along the pupillary margin (yellow arrow) in an 86-year-old pseudophakic patient who attended CFEH.
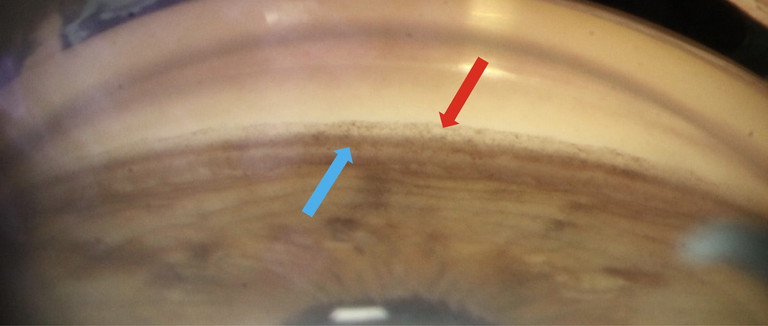
Figure 3. Gonioscopy findings of the inferior angle in a 74-year-old female patient with pseudoexfoliation syndrome who attended CFEH. There is increased pigmentation of the trabecular meshwork (blue arrow) and Sampolesi’s line may be variably present (red arrow).
A 2018 meta-analysis by Chung et al. reported an elevated risk of cardiovascular disease (OR 1.61) and cerebrovascular events (OR 1.76) in individuals with PXF.23 Proposed mechanisms include elastotic degeneration of vascular walls, endothelial dysfunction, and increased levels of homocysteine, a known contributor to vascular injury.25 Histopathological parallels between pseudoexfoliative material and elastotic changes in the aortic wall further support a vascular component.33
PXF has also been associated with increased carotid artery stiffness, reduced brachial artery endothelial function, and a potential genetic link with spontaneous cervical artery dissection via LOXL1 gene polymorphisms.34 While some studies report an association with aortic aneurysms and atherosclerosis, findings remain inconsistent.35
Cerebrovascular implications include a higher incidence of transient ischaemic attacks, strokes, and white matter hyperintensities on magnetic resonance imaging (MRI).36 Some studies have also proposed a potential link with Alzheimer’s disease, though this association is yet to be definitively established.36
“PXF is increasingly recognised as a systemic disorder characterised by the extracellular accumulation of fibrillar material...”
Beyond vascular implications, systemic involvement may extend to pulmonary and auditory systems. PXF has been associated with obstructive airway diseases, such as asthma, as well as a higher prevalence of sensorineural hearing loss.37,38 A hypothesised mechanism includes shared embryological origins of the inner ear and anterior segment structures, or broader extracellular matrix dysfunction.38
These multisystem associations underscore the importance of recognising PXF as more than an isolated ocular disease. Its systemic involvement warrants a multidisciplinary approach to patient care, particularly in those with concomitant cardiovascular or neurologic risk factors. Additionally, optical coherence tomography angiography studies have demonstrated reduced ocular perfusion in PXF, particularly in cases of PXG, supporting the hypothesis of widespread microvascular compromise.39
CURRENT AND EMERGING TREATMENT STRATEGIES
The Olmsted County Study looked at the natural course of PXF and found that 44% required glaucoma therapy over a 15-year period.40 The primary therapeutic goal in PXG remains reduction of IOP, consistent with the management of POAG. Currently, no approved therapies target the formation or accumulation of PXF material directly. PXG may also progress independent of IOP, with compromised lamina cribrosa and reduced perfusion likely playing a part.19,41
Medical Management
First-line treatment typically involves prostaglandin analogues (PGAs), such as latanoprost and travoprost, or the prostamide bimatoprost, which enhance uveoscleral outflow.42 Emerging evidence suggests PGAs may also influence the underlying pathophysiology of PXG by increasing matrix metalloproteinase expression, resulting in improved degradation of extracellular matrix components.43 Preservative-free formulations may be preferable in patients with ocular surface disease, which is more prevalent in PXF.
Beta-blockers, such as timolol, are also used in first-line therapy; however, latanoprost may offer superior IOP reduction and more consistent diurnal control.44 Timolol is contraindicated in individuals with asthma or pulmonary comorbidities due to potential systemic adverse effects.
Monotherapy is often inadequate in PXG due to typically higher baseline IOP and aggressive disease course, with treatment escalation often being required.
Laser and Surgical Approaches
Selective laser trabeculoplasty (SLT) is a viable first-line or adjunct therapy in PXG and PXF ocular hypertension.45 Due to the typically increased trabecular meshwork pigmentation, energy titration is necessary to minimise the risk of IOP spikes. Repeat SLT may also be needed due to declining efficacy over time.45
Minimally invasive glaucoma surgery (MIGS), such as iStent implantation, offers additional options for patients with mild-to-moderate PXG.46 MIGS presents a favourable safety profile compared to traditional filtration procedures with comparable IOP lowering to POAG patients,46 however research specifically targeting PXG cohorts is limited.
When medical and laser therapies fail, surgical intervention may be required, with trabeculectomy remaining the gold standard. PXG patients have a higher rate of surgical intervention and are at increased risk for postoperative complications, including exaggerated inflammatory responses, fibrin formation, posterior synechiae, and significant endothelial cell loss.46 Careful perioperative planning is required in this population.
“This type of glaucoma is associated with higher intraocular pressure (IOP) levels, greater IOP fluctuation, more advanced optic nerve damage at diagnosis, as well as a faster rate of progression when compared to primary open angle glaucoma”
CONCLUSION AND CLINICAL PEARLS
Pseudoexfoliation syndrome is a common but often underdiagnosed condition with significant ocular and potential systemic implications. Early recognition is critical, particularly given its association with rapid glaucoma progression. Ongoing careful slit-lamp examination remains essential, including in pseudophakic patients, where residual signs may be subtle. Gonioscopy is indispensable for assessing angle configuration and identifying typical pigmentation or pseudoexfoliative deposits. As evidence continues to emerge, optical coherence tomography angiography may play a future role in detecting ocular perfusion deficits associated with vascular dysfunction in PXF.
Close follow-up is warranted due to the high risk of IOP elevation and glaucoma development. Six-monthly IOP monitoring is recommended; however, closer follow-ups may be required where indicated, given the typically aggressive course of glaucoma progression. Escalation of therapy should be timely if IOP control wanes or there is evidence of progression, with early consideration of surgical intervention in cases refractory to maximal medical or laser therapy.
Given the zonular instability and poor pupil dilation, early referral for cataract surgery is recommended, particularly in asymmetric or early-stage disease, where surgical risks may be lower. Preoperative planning should anticipate potential complications, including the need for capsular tension devices or advanced IOL designs.
Patients may benefit from advice on smoking cessation, limiting caffeine and alcohol intake, UV protection, and adherence to an antioxidant-rich diet; however further research is required. Finally, clinicians should remain vigilant to the systemic nature of PXF. Cardiovascular evaluation should be encouraged in all patients, particularly those without recent primary care review. Recognising pseudoexfoliation as a multisystem disorder is key to delivering holistic and appropriate patient care.
To earn your CPD hours from this article, visit mieducation.com/more-than-meets-the-eye-pseudoexfoliation-syndrome.
References available at mieducation.com.

Nicole Lawson BOptom (Hons) BSci (Vis Sci) OACAP-G is a Senior Staff Optometrist at Centre for Eye Health and a Clinical Optometrist Supervisor at the UNSW School of Optometry.
Ms Lawson has a particular interest in the early detection and intervention of ocular pathologies, recently completing the Optometry Australia Advanced Practice Recognition Program in Glaucoma, which formally recognises a high level of skill and knowledge in the diagnosis, management, and co-management of glaucoma patients.