mieducation
Putting the Spotlight on
Retinal Vein Occlusions
Retinal vein occlusions (RVOs) are one of the most common presentations to eye care professionals.
Some of you may have read a recent article in Clinical and Experimental Optometry that discussed an interesting association between branch retinal vein occlusions (BRVO) and wind musicians, that I co-authored with an optometrist colleague of mine.1
When writing the article, we considered that one of the interesting facts about RVOs is that there is much about their aetiology that is still unknown. This, of course, becomes a little academic when we are able to diagnose and manage this condition with reasonably good visual outcomes.
WRITER Dr Christolyn Raj
LEARNING OBJECTIVES
On completion of this CPD activity, participants should:
1. Appreciate the complex underlying aetiology of RVOs,
2. Improve history taking to elicit potential risk factors,
3. Understand the clinical definition of central and branch retinal occlusion and its sequelae, and
4. Be aware of current evidence-based management strategies and potential future therapeutic interventions for RVO.
AETIOLOGY OF RETINAL VEIN OCCLUSIONS
RVOs represent the second leading cause of retinal vascular disorders.2 The underlying mechanism for occlusion is likely related, at least in part, to one or all of the features described by Virchow’s triad.3 Essentially, this refers to blood vessel wall trauma, stasis of blood flow, and a hypercoagulable state. It is believed that increased arteriolar rigidity as we age may increase the risk of compression at arteriovenous crossings. This, combined with systemic hypertension and atherosclerosis, can exacerbate arteriolar sclerosis.4 In theory, it is plausible, therefore, that uncontrolled or untreated systemic hypertension in a narrow older blood vessel lumen can potentiate trauma contributing to occlusion. Similarly, it has also been reported that fluctuations in systemic blood pressure, as a result of repetitive Valsalva manoeuvre during singing in high registers or playing high resistance wind instruments, could also cause trauma to the blood vessel wall.5 Stasis of blood flow is another factor that has been linked to RVOs, in particular external factors that may increase a thrombophilia risk include smoking, hormone replacement therapy (HRT), and the oral contraceptive pill. Further research into blood dyscrasias involving platelet and other clotting factor anomalies have also been linked to central retinal vein occlusions (CRVO).6,7
As a result of these associations in the literature, we have refined our clinical skills to often enquire about these specific factors during our history taking when a patient presents with an RVO. However, we can still be a little more precise in our assessment here.
IMPROVING HISTORY TAKING
While we commonly enquire about systemic hypertension, what we really want to know is: How well controlled is it? How frequently is it checked? In addition, we should ask if there are a number of medications involved as this is usually indicative of challenging control. Furthermore, it is important to note if a patient is symptomatic of these medications, as this may suggest under or overtreatment, which may predispose them to developing an RVO.
Considering thrombophilia risk, it may be prudent to ask about previous history of blood clotting, such as deep vein thrombosis (DVT) or pulmonary embolism, chemotherapy and/or radiotherapy, as well as commonly known clotting disorders, including Factor 5 Leiden or Protein S or C deficiency.
It is reasonable to also consider a patient’s relevant social history, in particular smoking habits and duration of smoking, and strenuous activities such as intense cardio fitness and cardio-weight training, which may impact on hypertension. Finally, as our small case series highlighted, playing a high resistance wind-instrument (trombone, French horn, trumpet) or intensive singing at high registers (soprano, mezzo-soprano, tenor) may be a plausible risk association in pre-disposed individuals.
Not only will spending some time here help to elucidate potential risk factors for a patient presenting with an RVO, it will also provide you with a concise vignette of the patient sitting in front of you, allowing you to uniquely tailor a management plan to them.
CLINICAL ASPECTS OF RETINAL VEIN OCCLUSION
RVO has been broadly categorised in two types: CRVO, which has a reported prevalence of 0.1–0.4%, and branch retinal vein occlusion (BRVO), which has a prevalence of 0.6–1.2 %.8,9 However, within these two categories are further subclassifications, which include a hemiretinal RVO as well as macular BRVO (Figure 1). It is also important to note that RVO may occur in concert with a retinal artery occlusion and this should be looked for.
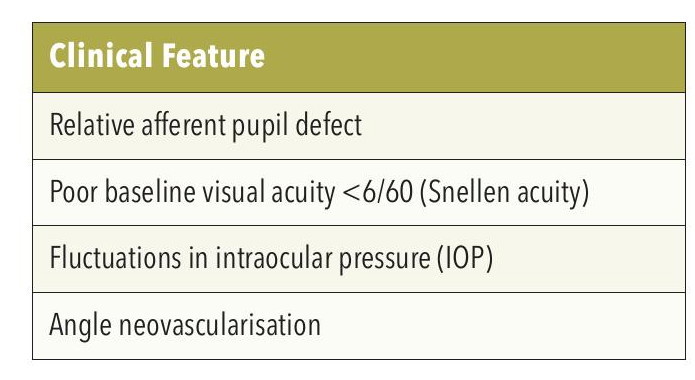
Table 1. Features of ischemic retinal vein occlusion.
In CRVO, the point of occlusion is at the level of the lamina cribrosa of the optic disc, though the severity of the occlusion can vary, resulting in retinal non-perfusion of varying degrees. There has been a reported conversion from non-ischemic to ischemic CRVO in 13–30% of cases.10
In BRVO, the localisation of the occlusion is usually the arterio-venous crossing along the course of a major retinal venous branch with the corresponding retinal quadrant/s being affected by the non-perfusion.
In a macular BRVO, the occlusion involves a small venous vessel draining a specific region of the macula.
In some cases, the subtleties of a RVO can be challenging to detect at the slit lamp.
A fundus fluorescein angiogram (FFA) or OCT-angiogram (OCT-A) will often be needed to appreciate the extent of the RVO (Figure 2).11
A hemiretinal vein occlusion refers to the involvement of one hemisphere of the retinal fundus. It occurs as a result of occlusion involving one of the two trunks of an abnormally split central retinal vein. This anomaly exists in 20% of cases.12
Making a clinical diagnosis of ischemic versus non ischemic RVO is essential in managing these patients but can be a challenge. Table 1 outlines typical features seen in ischaemic CRVO, which can help with this diagnosis. Some of these signs, such as baseline visual acuity and presence of a relative afferent pupillary defect (RAPD), are best appreciated away from the retinal fundus.
Clinical signs of ischemia in the retina can be challenging and may be identified by the presence of multiple, deep intraretinal haemorrhages and cotton wool spots. In more chronic cases, attenuated and sclerosed vessels may be present (Figure 3).
Essentially, an FFA or OCT-A is required to assess the extent of capillary non-perfusion seen in ischemic RVO. It has been reported that capillary non-perfusion to the extent of greater than 10 disc diameters in CRVO suggests a high risk of neovascularisation. In BRVO, studies report that capillary nonperfusion of at least five disc diameters has a high risk of neovascularisation.13,14
The risk of development of RVO in the fellow eye should always be considered in every patient. The Central Vein Occlusion Study (CVOS) group reported a 0.9% increase per year in the risk of developing a RVO in the fellow eye in patients presenting with unilateral CRVO. In contrast, the risk of developing BRVO in the fellow eye is much higher, reported in the order of 7–10%.15,16
“While we commonly enquire about systemic hypertension, what we really want to know is: How well controlled is it? How frequently is it checked?”
MANAGEMENT OF RETINAL VEIN OCCLUSION
While there currently exists no safe or effective treatment to reverse the vascular occlusion, these therapies target the sequelae of the disease process, mainly macular oedema, with the aim of preventing further visual loss.
The advent of anti-vascular endothelial growth factor (anti-VEGF) and biosimilar intravitreal agents has revolutionised the treatment of RVO. While anti-VEGF treatments have been the standard of care for the past decade, biosimilar agents are now emerging as equally effective treatments.
Two large pioneering studies that initially investigated anti-VEGF therapy (ranibizumab or aflibercept) in BRVO were the BRAVO and VIBRANT studies. BRAVO compared two different dosing regimens of ranibizumab. It showed that at six months, ranibizumabtreated eyes gained about 16–18 letters, compared to the no treatment group, and maintained this at 12 months. The VIBRANT study compared intravitreal aflibercept to focal macular laser and reported aflibercepttreated eyes improved by 17.1 letters compared to those treated with laser alone at 52 weeks.17,18
Similar studies investigating anti-VEGF therapy outcomes in CRVO have been the CRUISE, COPERNICUS, and GALILEO studies. The CRUISE study compared two different dosing regimens of ranibizumab with better visual outcomes than the no treatment group. The COPERNICUS and GALILEO studies compared six monthly intravitreal aflibercept followed by, as required, pro re nata injections to no treatment. These studies reported that up to 60% of aflibercept-treated eyes gained at least 15 letters of vision compared with 12–22% of eyes that did not receive treatment.19
More recently, the QUASAR study investigated the safety and efficacy of a higher dosage of aflibercept 8 mg (Eylea HD) for macular oedema secondary to RVO. The study concluded that the higher dose of Eylea administered at a longer interval of eight weeks was as effective as lower dose Eylea 4 mg administered monthly, in maintaining visual gains in patients with macular oedema from RVO.20
With the emergence of biosimilar agents – in particular those targeting both VEGF and angiopoietin 2, intravitreal faricimab has shown to be as effective as alflibercept in clinician trials for treatment of macular oedema associated with both BRVO (BALATON) and CRVO (COMINO). Long-term real-world data is still needed to accurately determine how these agents will shape future management of patients with this condition.21
Intravitreal corticosteroids have also been investigated. The SCORE-BRVO study compared the safety and effectiveness of intravitreal triamcinolone to laser and reported that, due to the significant increase in adverse effects of intraocular pressure rise and cataract, intravitreal triamcinolone should not be advocated as first-line treatment. It continues to serve as a second-line agent where treatment with anti-VEGF is contraindicated. A dexamethasone implant (Ozurdex) was evaluated in the GENEVA study for all RVOs and reported that patients treated with the implant had overall better visual gains at the 90-day period with minimal side effects.22,23
Despite active treatment, the secondary thief of sight in RVO is ischemia, which can lead to dangerous sequelae; namely retinal neovascularisation and secondary glaucoma.
The mainstay of treatment here is pan-retinal laser photocoagulation (PRP), which destroys the ischemic retina, thereby downregulating inflammatory mediators and reducing the drive for neovascularisation. It is important to institute a close follow-up plan for patients with ischemic changes or a high index of suspicion to develop ischemia. In cases of ischemic BRVO, it has been suggested that follow-up visits should be scheduled every three to four months for the first 24 months, looking to exclude ocular neovascularisation. In cases of ischæmic CRVO, monthly followup is advised for the first six months and then three monthly for one to three years.24
“The advent of antivascular endothelial growth factor (anti-VEGF) and biosimilar intravitreal agents has revolutionised the treatment of RVO”
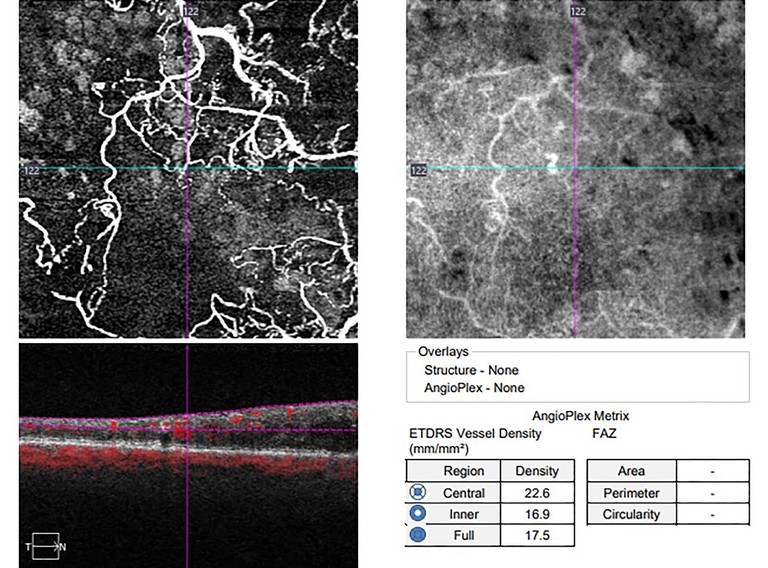
Figure 2. Optical coherence tomography angiogram (OCT-A) showing areas of retinal ischemia (dark regions) associated with a hemiretinal vein occlusion.
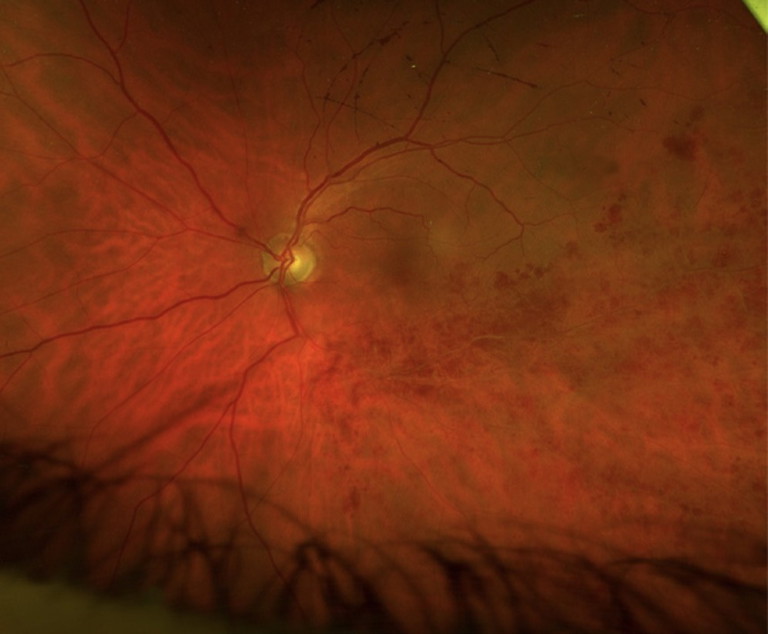
Branch retinal vein occlusion with ischemic changes in inferior quadrants (deep retinal haemorrhages, sclerosed arterioles).
“As much as we would like to follow a simple protocol to manage RVOs, the truth is that every patient case will be unique”
FUTURE DIRECTIONS
While still in their infancy, there are several clinical trials focussed on delivering more effective strategies for managing this disease. Other therapies under investigation include novel delivery systems such as a port delivery system for anti-VEGF and intravitreal hydrogels, and a laser-induced shunt between the retinal and choroidal circulation to bypass the vein occlusion.
In addition, a gene therapy targeting VEGF-Aproduction using an adenoviral vector to deliver an anti-VEGF gene to retinal pigment epithelium cells, so they can in turn express VEGF-A antibodies, is currently being trialled for patients with RVO-related maculopathy.25-27
REMEMBER, NOT ALL CASES ARE TEXTBOOK
As much as we would like to follow a simple protocol to manage RVOs, the truth is that every patient case will be unique. Some patients will show a rapid response to treatment, others will be slower, still others may need constant changes in their drug regimen to achieve stability. The visual prognosis for nonischemic RVOs, though overall positive, is still variable. For this reason, I make it a point to inform patients that the main aim of treatment is to achieve stability in their vision rather than promise improvement.
A particularly interesting case of mine; a 50-year-old male presenting with a nonischemic BRVO who achieved 6/6 vision after three months of treatment and remained stable up to 24 months, then developed ischemic changes. He subsequently required PRP and further intense anti-VEGF therapy to maintain his vision. This is just one case that highlights the important fact that RVOs are chronic disease entities that may continue to evolve and, as such, need our regular surveillance. Be wary that even cases that initially appear to be most stable can suddenly change course.
To earn your CPD hours from this article, visit mieducation.com/putting-the-spotlight-on-retinalvein-occlusions.
References
1. Raj C, Gutteridge I. Branch retinal vein occlusion and Valsalva manoeuvre in wind instrument players and singers. Clin Exp Optom. 2025 Jan 13:1-4. doi: 10.1080/08164622.2024.2448234.
2. Romano F, Lamanna F; Invernizzi A, et al. Update on retinal vein occlusion. Asia Pac J Ophthalmol (Phila). 2023 Mar-Apr 01;12(2):196-210. doi: 10.1097/APO.0000000000000598.
3. Chung I, Lip GY. Virchow’s triad revisited: blood constituents. Pathophysiol Haemost Thromb. 2003 Sep-2004 Dec;33(5-6):449-54. doi: 10.1159/000083844.
4. Oh T, Han K, Kim SW, et al., Hypertension as a risk factor for retinal vein occlusion in menopausal women: A nationwide Korean population-based study. Medicine (Baltimore). 2021;100(43):e27628. doi: 10.1097/MD.0000000000027628.
5. Aydin P, Oram O, Akman A, Dursun D. Effect of wind instrument playing on intraocular pressure. J Glaucoma. 2000;9(4):322-324. doi: 10.1097/00061198-200008000-00007.
6. Fegan, C. Central retinal vein occlusion and thrombophilia. Eye. 2022;16:98-106. doi: 10.1038/sj.eye.6700040.
7. Rath E, Frank R, Shin D, Kim C. Risk factors for retinal vein thrombosis: a case-control study. Ophthalmology 1992;99(4):509-514. doi: 10.1016/s0161-6420(92)31940-2.
8. Rogers S, McIntosh R, Wong TY, et al; International Eye Disease Consortium. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010 Feb;117(2):313-9.e1. doi: 10.1016/j.ophtha.2009.07.017.
9. Klein R, Moss SE, Meuer SM, Klein BE. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol. 2008;126(4):513-518. doi: 10.1001/archopht.126.4.513.
10. Jonas J, Paques M, Monés J, Glacet-Bernard A. Retinal vein occlusions. Dev Ophthalmol. 2010;47:111-135. doi: 10.1159/000320076.
11. Joffe L, Goldberg RE, Magargal LE, Annesley WH. Macular branch vein occlusion. Ophthalmology. 1980;87(2):91-98. doi: 10.1016/s0161-6420(80)35271-8.
12. Hayreh S, Hayreh M. Hemi-central retinal vein occulsion. Pathogenesis, clinical features, and natural history. Arch Ophthalmol. 1980;98(9):1600-1609. doi: 10.1001/archopht.1980.01020040452011.
13. Nicholson L, Vazquez-Alfageme C, Sivaprasad S, et al. Retinal nonperfusion in the posterior pole is associated with increased risk of neovascularization in central retinal vein occlusion. Am J Ophthalmol. 2017;182:118-125. doi: 10.1016/j.ajo.2017.07.015.
14. Yasuda Y, Hirano Y, Ogura Y, et al. Peripheral microvascular abnormalities detected by wide-field fluorescein angiography in eyes with branch retinal vein occlusion. Ophthalmic Res. 2019;61(2):107-114. doi: 10.1159/000488496.
15. Natural history and clinical management of central retinal vein occlusion. The Central Vein Occlusion Study Group. Arch Ophthalmol. 1997;115(4):486-491. doi: 10.1001/archopht.1997.01100150488006. Erratum in: Arch Ophthalmol 1997 Oct;115(10):1275.
16. Rogers SL, McIntosh RL, Wong TY, et al. Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117(6):1094-1101.e5. doi: 10.1016/j.ophtha.2010.01.058.
17. Clark WL, Boyer DS, Campochiaro PA, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: 52-week results of the VIBRANT Study. Ophthalmology. 2016;123(2):330-336. doi: 10.1016/j.ophtha.2015.09.035.
18. Campochiaro PA, Heier JS, Rubio RG, et al; BRAVO Investigators. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1102-1112.e1. doi: 10.1016/j.ophtha.2010.02.021.
19. Pielen A, Clark WL, Haller JA, et al. Integrated results from the COPERNICUS and GALILEO studies. Clin Ophthalmol. 2017;11:1533-1540. doi: 10.2147/OPTH.S140665.
20. Eylea HD (aflibercept) injection 8 mg phase 3 trial meets primary endpoint showing improved vision with extended dosing intervals in patients with macular edema following retinal vein occlusion. Regeneron Pharmaceuticals Inc. December 17, 2025.
21. Hattenbach LO, Abreu F, Arrisi P, Basu K et al. BALATON and COMINO: Phase III randomized clinical trials of faricimab for retinal vein occlusion: Study design and rationale. Ophthalmol Sci. 2023 Mar 27;3(3):100302. doi: 10.1016/j.xops.2023.100302.
22. Scott IU, Ip MS, Tolentino M, et al; SCORE Study Research Group. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol. 2009;127(9):1115-1128. doi: 10.1001/archophthalmol.2009.233. Erratum in: Arch Ophthalmol. 2009 Dec;127(12):1655.
23. Haller JA, Bandello F, Whitcup SM, et al; OZURDEX GENEVA Study Group. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117(6):1134-1146.e3. doi: 10.1016/j.ophtha.2010.03.032.
24. Nicholson L, Talks S, Sivaprasad S, et al. Retinal vein occlusion (RVO) guideline: executive summary. Eye (Lond). 2022;36(5):909-912. doi: 10.1038/s41433-022-02007-4.
25. Ranade SV, Wieland MR, Stewart JM, et al. The port delivery system with ranibizumab: a new paradigm for long-acting retinal drug delivery. Drug Deliv. 2022;29(1):1326-1334. doi: 10.1080/10717544.2022.2069301.
26. McAllister IL. Chorioretinal anastomosis for central retinal vein occlusion: a review of its development, technique, complications, and role in management. Asia Pac J Ophthalmol (Phila). 2020;9(3):239-249. doi: 10.1097/APO.0000000000000286.
27. Kim JE, Glassman AR, Sun JK, et al; DRCR Retina Network. A randomized trial of photobiomodulation therapy for center-involved diabetic macular edema with good visual acuity (Protocol AE). Ophthalmol Retina. 2022;6(4):298-307. doi: 10.1016/j.oret.2021.10.003.

Dr Christolyn Raj MBBS (Hons) MMED MPH FRANZCO is a Melbourne-based ophthalmologist. Dr Raj is a Fellow of the Royal Australian and New Zealand College of Ophthalmologists and the American Association of Ophthalmologists.
Dr Raj is experienced in the treatment of retinal vascular disease and has established herself as a key figure in the field of retinal medicine. She has published a chapter on diabetic retinopathy in the Textbook of Vascular Medicine, (Springer 2019). Her current research affiliation within the University of Melbourne is looking into novel therapies for the early treatment of diabetic maculopathy. Dr Raj's collaborative research discussed in this article1 was recently presented at the European Ophthalmology Congress 2025.