mieducation
What’s In, What’s Out?
DEWS III and the Future of Dry Eye Care
In the ever-evolving field of ocular surface disease, staying current is a matter of clinical necessity. With the recent release of the TFOS DEWS III (Dry Eye Workshop III) report,1,2 our industry is witnessing a significant recalibration of how we diagnose, classify, and manage dry eye disease. The DEWS III report represents a monumental collaboration: over 1,000 peer-reviewed publications synthesised, critically evaluated, and distilled into practical guidelines by 18 leading experts across multiple continents. This resource allows us to tailor a more nuanced, evidence-based roadmap for delivering best-practice dry eye care in 2025 and beyond.
WRITERS Jason Holland and Shaina Zheng
LEARNING OBJECTIVES
On completion of this CPD activity, participants should:
1. Be aware of the TFOS DEWS III report findings,
2. Understand key updates in DEWS III vs DEWS II,
3. Realise how to implement these findings in clinical practice, and
4. Understand how these changes will enhance patient outcomes.
FROM DEWS II TO DEWS III: EVOLUTION, NOT REVOLUTION
When DEWS II launched in 2017,3 it marked a paradigm shift from earlier definitions, emphasising tear film instability and inflammation. Yet in clinical practice, the framework often defaulted to a binary: aqueous-deficient vs evaporative dry eye. DEWS III doesn’t discard this entirely, but it pushes us further toward a layered, subtype driven approach.
The biggest structural update is the reclassification of dry eye into aetiological drivers, grouped under three diagnostic domains:
1. Tear film deficiencies: lipid, aqueous, and mucin (Figure 1),
2. Eyelid anomalies: blinking/lid closure, anterior blepharitis and meibomian gland dysfunction (Figure 2), and
3. Ocular surface abnormalities: anatomical misalignment, neural dysfunction, cellular damage, and inflammation (Figure 3).
This departure from the aqueous vs evaporative dichotomy allows for greater clinical flexibility. Patients often present with overlapping features. DEWS III helps us identify the dominant mechanisms and tailor interventions accordingly. It places the patient’s unique constellation of signs and symptoms at the heart of the diagnostic process, matching this with the evidence-identified mechanism of action of treatment and therapies. The study also emphasises shared decision making with the patient to help enhance patient outcomes and quality of life.
WHAT’S IN?
Subtype Based Management
No more generic eye drops for everyone. With defined subtypes, DEWS III introduces targeted treatment protocols. This modular strategy promotes matching therapy to mechanism, increasing the likelihood of symptom resolution and treatment adherence.
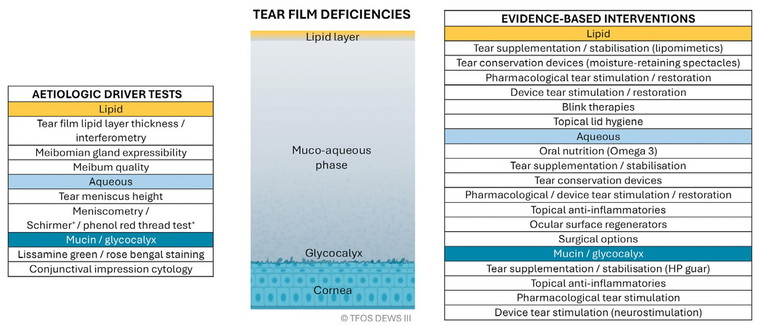
Figure 1. Tear film deficiencies.1
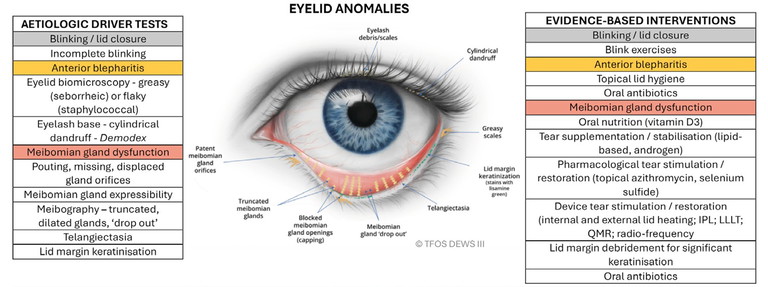
Figure 2. Eyelid anomalies.1
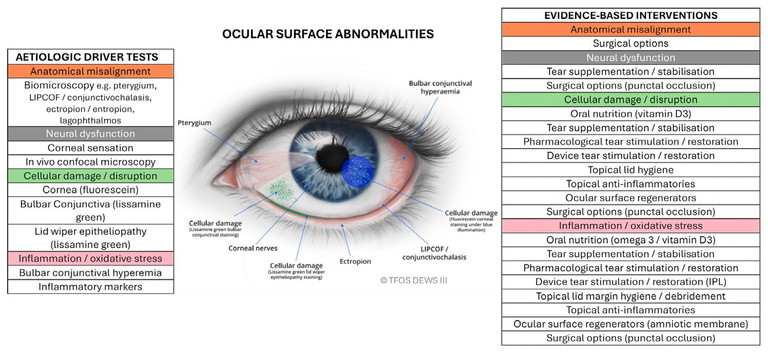
Figure 3. Ocular surface abnormalities.1
This is particularly crucial for patients who have tried multiple treatments with limited success, often because their underlying aetiology was never correctly identified.
A meta-analysis of 18 studies comparing hyaluronic acid (HA) to non-HA containing eye drops showed superiority in HA containing lubricants in improving patient symptoms and ocular staining.
It has also been suggested that combination eye drops outperform single polymer eye drops. One randomised control trial (RCT) comparing drops containing carboxymethylcellulose (CMC) to CMC and HA found superior improvement in ocular surface disease index (OSDI) and tear film break-up time (TBUT) in the HA and CMC group.
Lipid containing eye drops are suggested for patients with a reduced or unstable lipid layer. Various formulations are available in Australia:
• Castor oil – Optive Advanced, Cequa,
• Sesame oil and polyoxyethylene castor oil – Rohto Dry Aid,
• Mineral oil – Cationorm, Ikervis,
• Mineral oil and phospholipids – Systane Complete, Systane Balance,
• Liposomal phospholipid spray – Tears Again/Optrex ActiMist, and
• Tear stabiliser perfluorohexyloctane – NovaTears.
NovaTears can be used in isolation but is often layered with other topical lubricants. One study comparing Cationorm to NovaTears found that the lipid layer thickness (LLT) and higher order aberrations improved immediately after the instillation of Cationorm while NovaTears took 12 weeks to increase LLT and provided no improvement to higher order aberrations. Topical perfluorohexyloctane reduces the corneal surface temperature and increases the activity of TRMP8 thermoreceptors. This response can increase lacrimation and blinking, and reduce discomfort and pain.
Dosage and duration of eye drops is often variable between practitioners. A systematic review of 64 RCTs found good evidence that symptoms should improve within a month for four times a day dosing. However, dry eye signs can take four months to improve.
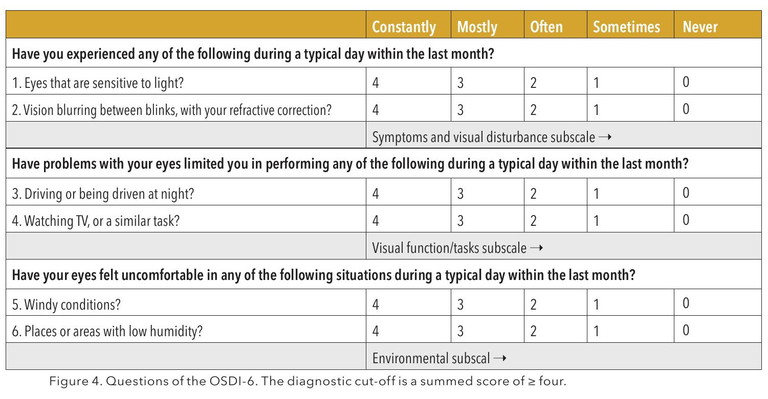
OSDI-6 Questionnaire
A simplified, validated six-question version of the OSDI now serves as the recommended first-line screening tool.2 It’s faster, scalable, and easily integrated into any practice. Importantly, it acknowledges that symptoms, not just clinical signs, must guide decision making.
Diagnostic Methodology
The emphasis for dry eye diagnosis has clearly shifted to positive symptomatology OSDI -6≥4, as well as one of: non-invasive TBUT (NIBUT) <10secs; as well as tear film osmolarity ≥308 mOsm/L in either eye; or interocular difference >8 mOsm/L (cut-offs established with the TearLab device only); and ocular surface staining of >5 corneal or >9 conjunctival punctate spots or lid margin staining of ≥2 mm length and ≥25% width, following the instillation of both fluorescein and lissamine green dyes. Technologies like the Oculus Keratograph and TearLab are increasingly regarded as standard-of-care. Objective imaging supports clear communication with patients and tracks subtle improvements over time.
Lifestyle as a Therapeutic Target
DEWS III repositions lifestyle factors as central to both diagnosis and ongoing care. Screen time, environmental triggers, diet, cosmetics, and contact lens wear are now seen as modifiable risk factors deserving active management. Discussing daily habits with patients allows for individualised education and empowers them to take ownership of their condition.
Patient Focussed Outcome Measures
There is growing emphasis on patient reported outcomes, not just clinician observed signs. Tools like the OSDI-6questionnaires are central to tracking progress and calibrating treatment. Patients should feel heard, understood, and engaged in their care journey. This builds trust and boosts long-term compliance.
WHAT’S OUT?
The Aqueous vs Evaporative Binary
While not irrelevant, this model is now considered overly simplistic. Many patients don’t fit cleanly into one category. DEWS III encourages nuanced, multifactorial diagnosis.
One Size Fits All Prescribing
Lubricant drops without subtype consideration are increasingly discouraged. The days of a ‘trial and error’ approach are giving way to precision guided therapy that respects patient individuality.
Neglecting Blink Metrics
Blink rate and completeness were often overlooked in the past. DEWS III integrates blink analysis into its core diagnostic and management framework. This is especially important for device users, whose blink rates are often suppressed. Incomplete blinking has also been found to have a 2.2-fold increased risk for dry eye disease (DED). Patients exhibiting incomplete blinking have higher OSDI scores, more significant meibomian gland (MG) drop out, and reduced LLT and NIBUT.
Ignoring Systemic and Lifestyle Influences
DEWS III places high importance on systemic medications, hormone status, and environmental factors. Best practice involves explaining the importance of lifestyle changes for treatment of dry eye disease.
Emerging Treatment Technologies
The therapeutic toolbox is expanding rapidly, and DEWS III recognises several innovations:
Lid heating devices. There has been an explosion of devices to heat the oil of the meibomian glands. Some have chemical thermal activation, some are heated via a microwave, and some are powered. The devices should heat to 40–41.5° and should be applied for 10 minutes once a day or five minutes twice a day, followed by gentle expression of the glands. Unfortunately, few studies exist on the commercially available devices in Australia. One study on the MGD Rx Bag found an improvement in LLT and NIBUT after two weeks of use.
Intense pulsed light (IPL). IPL involves the application of a series of non-coherent polychromatic light pulses to the periorbital region, producing selective photothermolysis of the irradiated tissue, leading to ablation and reduction of telangiectatic blood vessels around the eyelid margin.
“The biggest structural update is the reclassification of dry eye into aetiological drivers, grouped under three diagnostic domains”
IPL has been shown to reduce the signs and symptoms of DED, improve vision quality, increase the lipid layer thickness, and reduce dependency on artificial tears. One of the challenges when interpreting studies on IPL is the significant variation between IPL device design. Device variations can involve the light filter wavelength, treatment fluence, and exposure time. These variables also necessitate device specific protocols for optimum treatment outcomes. A review of 11 RCTs, published between 2015 and 2021 on 759 patients, found IPL improved NIBUT and TBUT but offered minimal improvement to dry eye symptoms. Another review covering 11 RCTs until 2022 on 1,842 participants found IPL improved symptoms (OSDI and Standardised Patient Evaluation of Eye Dryness (SPEED)) and increased tear stability (TBUT and NIBUT), but had no impact on corneal staining. The most recent systematic review of 13 papers on 931 subjects found TBUT and OSDI improved, but SPEED and corneal fluorescein staining (CFS) did not. Another variable is that most devices can only treat the lower eyelid, cheek, and tragus area. One study compared treating lower only or both eyelids and found patients who had both eyelids treated showed more improvement in parameters and higher satisfaction with treatment. IPL has also been shown in one RCT to reduce tear film cytokine levels.
Low-level light therapy (LLLT). This therapy involves the application of low-power, high-fluence monochromatic or quasimonochromatic light from light-emitting diodes through a wide array of wavelengths. LLLT is believed to work via the process of photobiomodulation.
Few studies exist assessing Red LLLT in isolation. One RCT found, after two treatments per week for three weeks, an increase in Schirmer score, an improvement to sodium fluorescein (NaFl) and lissamine green staining, and upper lid MG drop out score. Another study on 30 subjects receiving a 15-minute treatment every week for three weeks found significant improvements in tear meniscus height and Schirmer, NIBUT and LLT, OSDI, and meibum quality score.
A common question posed when starting a dry eye clinic is which technology is best: IPL or LLLT? One RCT of 40 patients compared Red LLLT and IPL finding both groups had an improvement in dry eye symptoms, with the LLLT group showing a more significant improvement along with an increase in tear volume.
Combining Red LLLT and IPL has also been investigated. One large retrospective study on 460 patients receiving 2–4 treatments 1–2 weeks apart found a significant reduction in OSDI and TBUT. MGD grading improved one step or more in 70% of subjects and two steps or more in 28% of subjects.
Blue LLLT has inherent antimicrobial properties due to the generation of reactive oxygen species on interaction with microbial cells, which can damage cellular components such as deoxyribonucleic acid, proteins, and lipids, leading to bacterial cell death.
Rexon-Eye. Quantum molecular resonance (QMR) is a unique electrotherapy system that regenerates ocular surface tissues.
A double blind RCT was conducted for two years on 40 subjects. The treatment arm had a 20-minute session every week for four weeks. Significant improvement was seen in OSDI, MGD scores, and corneal staining. No improvement was seen in TBUT or Schirmer score.
A case series on mixed dry eye patients found improvements in OSDI, NIBUT, corneal staining, MG parameters, and matrix metalloproteinase-9 (MMP-9) levels.
The authors of DEWS III felt that more research is required to fully understand the benefits of QMR for DED.
Radiofrequency (RF) therapy. Radiofrequency is an electromagnetic wave that uses an oscillatory field to charge particles within the target tissue, generating heat through friction between vibrating tissue particles.
There currently is limited evidence for the use of radiofrequency in the treatment of DED. One study of 31 subjects receiving IPL and
RF treatments with MG expression found significant improvements in the signs and symptoms of MGD, but there was no control group in the study.
Topical immunomodulators. Ciclosporine remains a mainstay for inflammatory driven DED, aqueous deficient, and cellular damage subtypes.
Over 600 peer reviewed papers have been published on ciclosporin A (CsA) and DED since 2017, however, only 60 have been RCTs. Thirty trials found significantly better efficacy with CsA, irrespective of dose or concentration, and the effect of CsA was comparable to tear supplements, fluorometholone 0.1%, tacrolimus 0.03%, and diquafosol 3% in 13 trials.
Lifitegrast is a lymphocyte function-associated antigen-1 (LFA-1) antagonist. A post hoc analysis from two RCTs found that signs and symptoms were more likely to improve in subjects with moderate to severe DED. A systemic review, however, found that 16% of patients reported dysgeusia, which led to a 7% discontinuation rate.
Autologous serum and biologic tears. For aqueous, neurotrophic, cellular damage, or inflammation DED presentations, blood-based eye drops represent an emerging treatment and come in a variety of options. Access and standardisation of preparation remain a challenge for patients. Platelet rich plasma (PRP) is a possible treatment option for patients with Sjogren’s disease.
“A simplified, validated six-question version of the OSDI now serves as the recommended firstline screening tool”
Pharmacological neuromodulation. Eye drops acting as TRPM8 agonists are in development. TRPM8 ion channels are stimulated by hyperosmolarity or a reduction in tear film temperature, as seen with evaporative DED. Stimulation of TRPM8 leads to increased signalling through the trigeminal nerve, which results in an increase in basal tear production. Menthol also acts on TRPM8 receptors and is now available as one ingredient of Rohto Dry Aid eye drops.
Importantly, DEWS III frames these technologies within an evidence-based context. Clinical use should be matched to subtype and mechanism. The goal is to treat the person behind the symptoms and tailor the approach.
BEYOND THE SURFACE: DRY EYE, MENTAL HEALTH, AND QOL
While DEWS III is rooted in precision diagnostics and pathophysiological clarity, it doesn’t overlook the emotional and psychological toll that dry eye disease can take. The report acknowledges the increasing evidence linking moderate to severe dry eye with symptoms of depression, anxiety, and decreased quality of life (QoL).
“ DEWS III... doesn’t overlook the emotional and psychological toll that dry eye disease can take ”
Patients with chronic ocular discomfort often experience a sense of frustration and hopelessness, particularly when their symptoms persist despite treatment, or when their complaints are dismissed due to minimal clinical signs. DEWS III encourages clinicians to recognise and validate this disconnect, and to treat patients with empathy, as much as efficacy.
Importantly, the emphasis on patient-reported outcome measures, such as the OSDI-6, goes beyond assessing physical discomfort. These tools help us understand the functional and emotional burden of dry eye: the sleep disruption, social withdrawal, work absenteeism, and mental fatigue that frequently accompany it.
By integrating mental health awareness into dry eye care, DEWS III reframes our role as clinicians, not just to treat the tear film, but to improve the patient’s overall wellbeing. We are reminded to listen without judgement, to screen for distress when appropriate, and to collaborate with other healthcare professionals when emotional health becomes a barrier to recovery.
BEST PRACTICE IN 2025: DEWS III INFORMED1
So, what does gold-standard dry eye care look like today?
1. Initial Visit:
o OSDI-6 questionnaire
o Perform NIBUT, tear osmolarity, and ocular surface staining,
o Evaluate blink rate and completeness, and o Discuss lifestyle and systemic health.
2. Diagnosis:
o If OSDI-6 ≥4, confirm dry eye with one or more abnormal tests (NBUT, osmolarity or staining), and
o Identify dominant aetiological driver(s). 3. Treatment: o Match therapy to subtype, o Integrate at home and in office treatments, o Address modifiable lifestyle factors, and
o Centre discussions around how symptoms affect the patient’s daily life, work, and wellbeing.
4. Follow-Up:
o Reassess both signs and symptoms every 4–12 weeks, and
o Adjust plan as needed, based on response and new findings.
THE TAKE-HOME MESSAGE
DEWS III represents more than just an update, it sets a new benchmark for dry eye care. It invites optometrists to deepen their diagnostic insight, tailor management strategies with greater precision, and approach dry eye with the recognition that it is a multifactorial, often complex disease that significantly impacts quality of life.
At its core, DEWS III is about understanding dry eye as a spectrum of subtypes, each with distinct drivers, and aligning treatment accordingly. This shift from a generic label to a nuanced diagnosis empowers us to deliver more targeted, effective care.
Dry eye is now firmly recognised as a disease entity, not just a symptom or inconvenience. DEWS III encourages clinicians to move beyond symptom suppression, and instead guide, support, and educate patients through a structured, evidence-based care journey.
To earn your CPD hours from this activity, visit: mieducation.com/whats-in-whats-out-dews-III-and the-future-of-dry-eye-care.
“There is growing emphasis on patient reported outcomes, not just clinician observed signs”
References
Note: All studies cited in this article are referenced in the following TFOS DEWS Reports:
1. Jones L, Craig JP, Wolffsohn JS, et al; + TFOS Collaborator Group. TFOS DEWS III management and therapy report, Am J Ophthalmol. 2025 Jun;279:289-386. doi: 10.1016/j.ajo.2025.05.039.
2. Wolffsohn JS, Benítez-Del-Castillo JM, Jones L, et al; + TFOS collaborator group. TFOS DEWS III diagnostic methodology, Am J Ophthal. 2025 May;279:387-450. doi: 10.1016/j.ajo.2025.05.033.
3. Tear Film and Ocular Surface Society, Dry eye redefined, TFOS DEWS II report, available at tfosdewsreport.org [accessed Sept 2025].

Jason Holland BAppSci (Hons) (Optom) PGOT CASA Co is the President of the Dry Eye Society and the National Director of Optometry for The Optical Superstore. He consults at Oculux by The Eye Health Centre in Brisbane, an advanced dry eye clinic that manages challenging patients using the latest technology.
The recipient of the Optometry Australia Queensland Optometrist of the Year award in 2018, he has lectured on contact lenses and dry eye in Australia and across South East Asia.

Shaina Zheng BOptom OcTherapeutics GAICD is a co-owner optometrist at Eyecare Plus Mermaid Beach in Queensland. She currently serves as the Vice President of the Dry Eye Society, where she drives professional education, clinical best practice initiatives, and industry collaboration. She is also the founder of Dry Eye Impact, an organisation dedicated to advancing patient-focussed dry eye care through innovation, compassion, and collaborative professional networks across Australia.