mieducation
Testing the Vision of Children
Childhood vision testing is an important task for all eye care practitioners, as good vision can sustain school achievement. Although there are few eye conditions to affect the sight of children, those that do can be serious. In this article, the authors explain how Melbourne Rapid Fields rapid vision test (MRF) software fills an important gap for child vision testing.
WRITERS Professor Viney Gupta, Dr Selwyn Prea, Associate Professor George Kong, and Professor Algis Vingrys
LEARNING OBJECTIVES
On completion of this CPD activity, participants should be able to:
1. Realise the various screening tests that can be conducted using MRF,
2. Recognise how it can be used in clinic and at home to monitor eye disease progression,
3. Understand the benefits of using MRF for screening children, and
4. Be aware of the evidence supporting MRF use.
MRF is registered with the Therapeutic Goods Administration (TGA) in Australia and represents a groundbreaking advancement for eye care testing. It offers online-based visual acuity and visual field testing that can be performed on any portable device, such as a tablet or computer, provided the monitors have UHD (or 4k) screens.
MRF increases the efficiency and portability of vision testing to individual clinics as well as beyond the clinic. By using web-based technology, patients can undergo these tests at home or clinicians can use the tests in outreach.
In adults, MRF is being used to monitor conditions such as glaucoma, age-related macular degeneration (AMD), macular oedema from diabetes or retinal vein occlusion (RVO), diabetic retinopathy (DR), and neurological conditions that affect vision or the visual field (including multiple sclerosis and optic neuritis). The acuity test suite can also be used to define reductions of vision due to cataract, corneal surgery, and contact lenses as well as brain disorders. In the following we will detail specific applications that pertain to children.
MRF software can also be successfully used to test the vision of children, for example, for school screening in low resource settings. It provides an easier and more controlled way of doing acuity testing than standard Snellen charts by adopting a simple matching task, which makes the test easy for children to perform. The fact that the test is done on a computer screen or tablet device means that the test brightness can be precisely controlled. The thing to watch for when using such tests is that reflections from the screen do not interfere with visibility.
Additionally, MRF can be used for home testing of vision in children with progressive eye diseases. That way parents can be alerted to an unexpected decrease in vision of the child and remedial action initiated early.
However, one of the more important changes to affect a child’s vision is the development of amblyopia. Amblyopia is the most common cause of preventable monocular vision loss in children and its detection and management is an important aspect for both the child and eye care providers.1
AMBLYOPIA AND ACUITY TESTING WITH MRF
Population surveys find that the prevalence of amblyopia varies around the world and has been found to range from 1–6% in India and about 2% in Australian pre-school children (age ≥ 30 months).1,2 Hyperopia, astigmatism, anisometropia, and strabismus are all major amblyogenic factors and infants who are premature, have a low gestational age or have first-degree relatives with amblyopia are four times more likely to express amblyopia.1,2 More importantly, early detection and correction of refractive error and anisometropia can reduce or prevent amblyopia entirely, so it is a condition that all optometrists need to actively look out for.3
Given that the visual system undergoes high levels of neural plasticity and organisation after birth, abnormal inputs from eyes due to strabismus or refractive error can affect cortical development, resulting in amblyopia. This makes early detection of such anomalies an important consideration. In some cases, these abnormal cortical changes can be reversed with targeted visual stimulation. Such knowledge accounts for the increased interest in the detection and management of amblyopia. Amblyopia treatment modalities include correction of refractive error, occlusion therapy, and penalisation, as well as newer methods that use eyeglasses or goggles to produce differential dichoptic images that reinvigorate brain connections. It is with this in mind that many paediatric patients present to optometrists to identify and manage their ‘lazy eye’.
The Paediatric Eye Disease Investigator Group found that correcting refractive error of children aged three to seven not only improved acuity by an average of three lines in the majority (77%) of cases, it reversed amblyopia in 27% children.3
Luminance Noise Masks
Identifying amblyopia rests in testing visual acuity and this can be challenging, depending on the age of the child. For this purpose, it would help if there was a simple test specific for cortical dysfunction and amblyopia. One candidate for such a test is a target presented in a luminance noise mask (Figure 1).5 Research, undertaken on humans and macaques with lesions in the brain, has shown that luminance noise masks impede the sensory processing of visual stimuli in these cases. Abnormal performance is found when visual noise is added to targets in several neurological conditions such as; stroke, migraine,4 visual snow, and schizophrenia.4,7,9,10 It can also be used to identify amblyopes.5
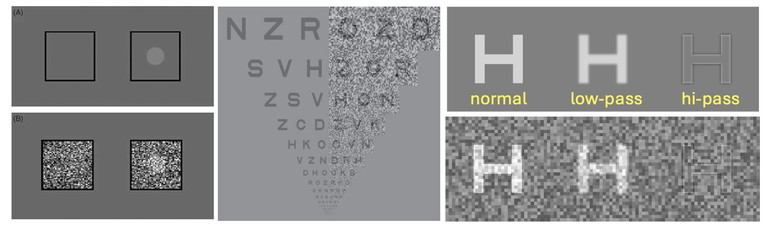
Figure 1. Using noise masks to test for cortical changes. Left: Threshold is determined for disc targets with and without different levels of a static noise mask. The subject’s task is to nominate which side (L or R) carries the disc. Centre: Fixed noise can be applied with an acuity chart, called the dual acuity chart. A person with normal vision should find that the noise mask has little impact on letter recognition. Right: The effect that static noise has on letter optotypes that are unfiltered (normal) or have been lowor high-pass filtered. The noise mask has greatest effect on the high-pass filtered target (lower right) as would be the case measuring the visual acuity limit. (L– R images taken from Webster et al.,4 Pelli et al.,5 and Hall et al.6 )
Testing with noise can be performed using a static noise mask (Figure 1). Figure 1 shows how the presence of static noise has little effect on normal (unfiltered) or low pass optotypes, but greatly obscures high pass filtered targets (bottom right panel) as would be seen at the limit of visual acuity. Research finds that static noise masks target magnocellular processes whereas dynamic noise jitter targets parvo-cellular pathways.6
The MRF includes an acuity optotype, immersed in dynamic luminance noise jitter (see Figure 2 for static version), specifically designed to target parvo-cellular cortical pathways.
The promising outcomes that we see with the acuity-in-noise target (Figure 3) are not found universally. The dual acuity chart (DAC) of Figure 1 was found to have limited sensitivity in distinguishing between normal and amblyopic eyes.11,12 Our 14 amblyopic eyes returned a sensitivity of 64%, close to that found with acute ischemic stroke (62%, Figure 2).
It is possible that the dynamic noise mask used by the MRF has a greater effect on visual processing than does the static mask of the DAC, where a poorer sensitivity was reported. Regardless, five of our 14 amblyopic observers gave normal outcomes (grey zone) and the others had modest reductions in acuity (two to three lines, Figure 3 right panel). So, it appears that the effect of noise masks is not profound in amblyopia and the test should be used as part of a diagnostic battery for amblyopia that includes standard visual acuity, acuity-in-noise, refractive error, cover test, and stereopsis.
PERIMETRY TESTING WITH MRF
Apart from assaying foveal spatial capacity with visual acuity, the testing of a child’s visual field poses unique challenges. Firstly, most visual field-testing devices have been designed from knowledge gained on adults, which might not apply to children. In particular, in testing children you will find that they return poorer reliability and give greater fixational instability owing to their reduced attentiveness.13-18 Hence, it is important to establish the suitability of approaches for the perimetric testing of young children.
The MRF perimetry test has been designed to cater for children due to its free-space presentation, ease of response, and voice interaction with the child. The short test durations (four to five minutes) are also favoured by children.
Perimetry is a desirable test for paediatric patients because it provides important information regarding the integrity of the eye, the optic nerve, and the visual pathways.13 It has been reported that standard automated perimetry is the most common form of visual field test used with children in hospitals in the United Kingdom, so the MRF is well placed for this purpose.13,14
The development of smart technology (phones and tablets) has produced engaging experiences across the educational and entertainment industries. These interactions are enjoyed by adults and children alike.
A commercial tablet device (iPad) provides an easy-to-use visual field test with the MRF software. This is partly because the voiceover audio that gives instructions, interacts with the child during testing, providing a sense of ‘gaming’ or ‘oversight’ in the testing process. Moreover, the perimetry task can be gamified further with instructions such as, ‘catch the firefly by tapping here when it flashes out of the corner of your eye’.
“ … abnormal inputs from eyes due to strabismus or refractive error can affect cortical development, resulting in amblyopia. This makes early detection of such anomalies an important consideration ”
EVALUATING SUITABILITY OF MRF FOR CHILDREN
The suitability of the MRF for the testing of paediatric cases was evaluated in a cohort of 16 children (aged seven to 16, mean 10 ±2.4 years) diagnosed with congenital glaucoma (32 eyes) by Prof Gupta and his team in New Delhi, India.
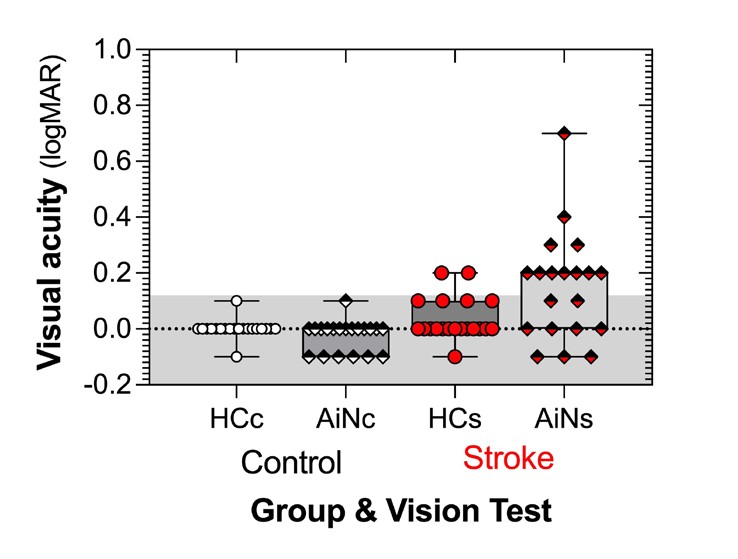
Figure 2. Acuity-in-noise is abnormal in many of our 20 patients who have had a recent stroke (within one week). (Adapted from Wijesundera et al.7 )
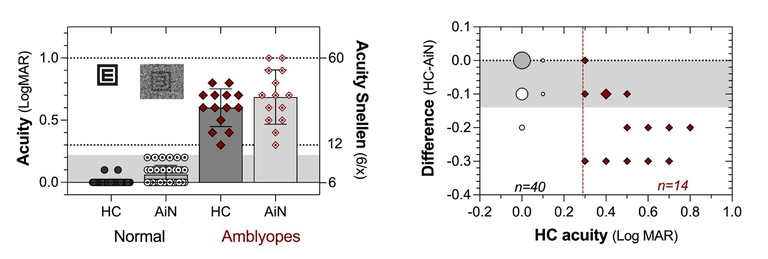
Figure 3. MRF masking the acuity target in dynamic luminance noise (AiN) produces a one-line difference from high contrast (HC) acuity in 40 normal eyes (grey area) but an average two-line difference in 14 amblyopic eyes (right panel).
This comparative clinical trial considered visual field outcomes returned by the MRF device to the standard bowl perimeter, Humphrey Field Analyser (HFA, ZEISS Meditech, Inc, Dublin, CA) used in the children’s clinic for diagnosis and management.
The usual practice of this clinic is for the clinical assistant to have the child perform a 24-2 SITA-standard test unless they felt that the child had limited attention, in which case they could adopt a 24-2 SITA-fast test. The trial has been reported in Translational Vision Science and Technology19 with key findings summarised below.
Children with the diagnosis of congenital glaucoma were identified and recruited to the trial. Diagnosis of congenital glaucoma required that the child had an intraocular pressure of >21 mmHg in the presence of at least one of the following:
1. An enlarged corneal diameter (≥11 mm at age one or ≥12 mm at any age),
2. Elongated axial length for age, or
3. A large cup-to-disc ratio (>0.6) or an asymmetry of more than 0.2 between eyes in the presence of focal rim thinning when the optic disc was of similar size.
All children with, or suspected of, such a diagnosis and their guardians were asked to consent to undertaking two perimetry tests, one at their first visit and another at a subsequent clinical follow-up, required for the management of their ocular condition. This follow-up visit ranged from two to 21 months after the first visit, consistent with real world clinical encounters for such clinical cases.
None of the children had ever performed perimetry testing before and the order of tests (MRF, HFA) was counterbalanced so that half did the MRF test first and the other half, the HFA first. All children were verbally instructed and given a one-minute practice trial with verbal encouragement to familiarise to the task. Finally, children were surveyed on their preferred testing format that they would like to use in the future.
COMPARISON RESULTS
Three children (five eyes (16%); aged eight, nine, and 13 years) could not complete the HFA SITA standard test at the first encounter but could do so in their fellow eye after having performed the MRF. It is likely that this order of testing provided some familiarity and learning to the perimetry testing task for these children, as the reverse was never seen.
Over both visits, we achieved 57 outcomes with the HFA perimeter (89% of 64 potential tests (two eyes x 16 children x two tests) and 62 outcomes (97%) with the MRF. A more concerning prospect was that three children (aged 13, 13, and 15) could not be retested on one or both eyes (16%) at the follow-up visit, despite a successful first test, indicating that a successful outcome does not guarantee future perimetric success in a child.
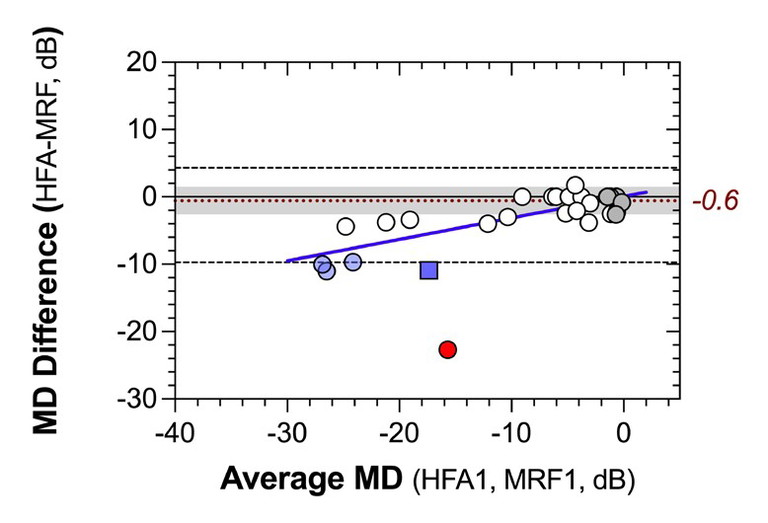
Figure 4. Concordance between MRF and HFA mean deviation plotted as a Bland-Altman plot. The average difference between devices in children with normal HFA MD is -0.6 dB and the coefficient of repeatability is 2.2 dB. The blue line shows proportionate bias in the data, likely due to the larger test spots used by MRF.
In the 32 eyes, six returned normal HFA mean deviations (MDs) with a suspect diagnosis, 12 had mild visual field loss, and 14 moderate to advanced loss. The average test time, over all eyes and visits for the MRF, was 5.5 ±1.9 minutes, similar to SITA-fast (5.4 ± 1.8 mins) with both being three minutes faster than SITA standard (8.5 ±2.2 mins).
A high level of concordance was found between the MD of the MRF and the HFA (ICC=0.91), indicating that clinicians can use the MRF MD as an accurate indication of HFA threshold in children. Most MRF outcomes were within ±5 dB of HFA MD except for some children with large losses (blue symbols) and one outlier (red symbol). Some children with large loss of sensitivity on the HFA returned greater departure from MRF values (Figure 4, blue data). Presence of this effect is indicated by the blue line in Figure 4 that is fit to that data and shows a proportionate bias (data departs more so from zero line at more negative values) with a slope of 0.32 ±0.05. This bias likely reflects higher thresholds in the peripheral locations of MRF due to the larger spots used in these regions. Notice that thresholds are greater for the MRF than for the HFA by about 10 dB (Figure 4, blue line HFA has more negative MD), providing a larger dynamic range for monitoring defects with MRF, especially in moderate to severe cases. One child (shown as the red circle in Figure 4) was an outlier producing a high HFA MD and a modest loss on MRF; it is likely that this child learned how to do perimetry on their first exposure with HFA and gave an improved outcome on the MRF (second exposure).
RETEST DATA
The retest data showed that children with no visual field loss (grey symbols) gave very repeatable outcomes on both tests, returning a coefficient of repeatability (CR) of 2.2 dB (shaded region, Figure 4). On the other hand, those with a field defect gave CR of about 7 dB for both HFA and MRF. Greater variability with visual field loss has been reported in adults as well as children by others.18
The CR is a useful measure of precision for clinicians as it defines the limit which contains 95% of repeat test outcomes. Our finding indicates that children with no visual field loss return highly reliable and repeatable outcomes on the MRF within 2.2 dB of the HFA value (greyed zone) and that if a child returns a normal MD value, they are likely to continue doing so in the future unless the disease progresses. On the other hand, those with a moderate field loss have high levels of variability and need to be retested several times to return a more precise threshold measure for future monitoring.
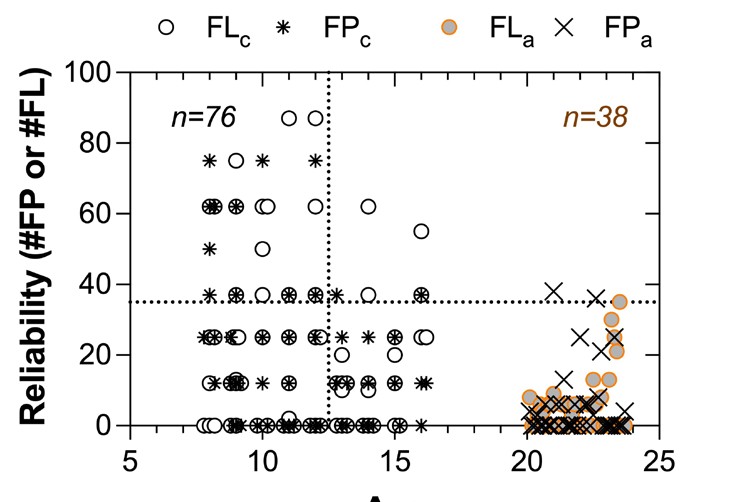
Figure 5. The number of MRF catch trials for fixation loss and false positive errors identified by the MRF as a function of age. Here we give the numbers of errors polled by the MRF trials for 76 eyes tested on children of various ages and contrast these to that obtained from 38 adults (university students > 20 years of age). Adult data shows that the 95% limit for errors is 37% but children make many more errors than adults, especially below age 12.5 years (vertical dotted line).
“ … standard automated perimetry is the most common form of visual field test used with children in hospitals in the United Kingdom, so the MRF is well placed for this purpose ”
Retesting four times will reduce the CR by a factor of two, to about 3.5 dB (7/2). Retesting can be undertaken in the clinic or by having children self-test at home under the supervision of a parent. This could be achieved as a cluster of tests just before the child returns for clinical review. However, the prospect for home-testing needs confirmation with a well conducted clinical trial.
CONCLUSION
As noted earlier, children return less reliable outcomes on the MRF, giving high levels of fixation loss and false positive responses, especially at younger ages. This does not appear device dependent as tests performed on the tablet and Humphrey devices identified about the same number of children with high fixation loss16 and high false positive rates at the two test sessions. That the poor reliability is not equipment specific is also evident by the high concordance between devices. Finally, reliability appears age dependent, as shown in Figure 5 where we plot from a larger data set of 76 eyes of children and 38 eyes of adults, mostly university students. Figure 5 shows that a criterion of 37% (horizontal dotted line) identifies 95% of reliable adult performance, whereas children can give highly unreliable outcomes, especially if younger than 12.5 years (vertical dotted line). However, the optimal cut-off to indicate reliable outcomes and the effect that unreliable indices can have on perimetric outcomes is currently debated and in need of further research.
The outcome of our survey after the second session of testing found that all children preferred the test experience of the MRF, reporting it easier to do and more agreeable than the HFA. Such findings will promote visual field testing in children using the MRF. The MRF should be considered as a test option for all children, and especially if they are reluctant to undertake visual field testing using routine bowl perimeters.
This article is sponsored by Glance Optical Pty Ltd the makers of the MRF vision software.
To earn your CPD hours from this article visit mieducation.com/testing-the-vision-of-children.

Professor Viney Gupta MD MBA is Professor of Ophthalmology at the Dr Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Science, in New Delhi, India. He has a special interest in congenital and juvenile glaucomas and did his Glaucoma Fellowship at Royal Victorian Eye and Ear Hospital in Melbourne.

Associate Professor George Kong MBBS BMedSci PhD FRANZCO is a consultant ophthalmologist at the Royal Victorian Eye and Ear Hospital in Melbourne, where he is a member of the Glaucoma Unit. He has extensive experience in comprehensive glaucoma management and complex glaucoma surgery. Assoc Prof Kong is a founder of Glance Optical P/L, which markets the Melbourne Rapid Fields – rapid vision test.

Professor Algis Vingrys PhD BSc(Optom) PGCertOcTher FARVO FAAO is an Emmeritus Professor in the Department of Optometry and Vision Sciences at the University of Melbourne. He specialises in clinical optometry and in researching early vision loss with eye and brain disorders. Prof Vingrys is a founder of Glance Optical P/L, which markets the Melbourne Rapid Fields – rapid vision test.

Dr Selwyn Prea BOptom MPhil (Optom) PhD is a Lecturer in Optometry and Vision Sciences at the University of Melbourne. He undertook his PhD research at the Glaucoma Unit at the Royal Victorian Eye and Ear Hospital in Melbourne.
References
1. Nambudiri S, Kumari PVG, Sudha V, Sinumol S. Amblyopia – An update. Kerala Journal of Ophthalmology 2021;33(1):14-21. doi: 10.4103/kjo.kjo_13_21.
2. Pai AS, Rose KA, Leone JF, et al. Amblyopia prevalence and risk factors in Australian preschool children.
Ophthalmology. Jan 2012;119(1):138-44. doi: 10.1016/j. ophtha.2011.06.024.
3. Cotter SA; Pediatric Eye Disease Investigator Group; Edwards AR, Wallace DK, et al. Treatment of anisometropic amblyopia in children with refractive correction. Ophthalmology. 2006; Ju 113 (6):895-903. doi: 10.1016/j. ophtha.2006.01.068.
4. Webster KE, Dickinson JE, Badcock DR, et al. Evidence for increased internal noise in migraineurs for contrast and shape processing. Cephalalgia. 2012 Jan; 32(2):125-39. doi: 10.1177/0333102411432725.
5. Pelli DG, Levi DM, Chung ST. Using visual noise to characterize amblyopic letter identification. J Vis. 2004 Oct 29;4(10):904-20. doi: 10.1167/4.10.6.
6. Hall CM, McAnany JJ. Luminance noise as a novel approach for measuring contrast sensitivity within the magnocellular and parvocellular pathways. J Vis. 2017 Jul 1;17(8):5. doi: 10.1167/17.8.5.
7. Wijesundera C, Vingrys AJ, Wijeratne T, Crewther SG. Acquired visual deficits independent of lesion site in acute stroke. Front Neurol. 2020;11:705. doi: 10.3389/ fneur.2020.00705.
8. Hayes RD, Merigan WH. Mechanisms of sensitivity loss due to visual cortex lesions in humans and macaques. Cereb Cortex. 2007 May;17(5):1117-28. doi: 10.1093/cercor/bhl021.
9 McKendrick AM, Chan YM, Tien M, et al. Behavioral measures of cortical hyperexcitability assessed in people who experience visual snow. Neurology. 2017 Mar 28;88(13):1243-1249. doi:
10.1212/WNL.0000000000003784. 10. Shoshina I, Isajeva E, Mukhitova Y, et al. The internal noise of the visual system and cognitive functions in schizophrenia. Procedia Computer Science. 2020;169:813820. doi: 10.1016/j.procs.2020.02.158.
11. Eaton KA. Efficacy of the Pelli-Levi dual acuity chart in diagnosing amblyopia. University of California, Berkeley; 2006.
12. Shelton JB, Harvey EM, Dobson V, Miller JM, Clifford CE. Performance of children with astigmatism– relatedamblyopia on the ‘Pelli–Levi dual acuity chart’. Invest. Ophthalmol. Vis. Sci. 2005;46(13):5644.
13. Walters BC, Rahi JS, Cumberland PM. Perimetry in children: Survey of current practices in the United Kingdom and Ireland. Ophthalmic Epidemiol. 2012 Dec;19(6):35863. doi: 10.3109/09286586.2012.718027.
14. Patel DE, Cumberland PM, Walters BC, et al. Study of Optimal Perimetric Testing In Children (OPTIC): Normative visual field values in children. Ophthalmology. 2015 Aug;122(8):1711-7. doi: 10.1016/j.ophtha.2015.04.038.
15 Patel DE, Cumberland PM, Walters BC, et al., OPTIC Study Group. Study of Optimal Perimetric Testing in Children (OPTIC): Feasibility, reliability and repeatability of perimetry in children. PLoS One. 2015;10(6):e0130895. doi: 10.1371/journal.pone.0130895.
16. Jones PR, Yasoubi N, Nardini M, Rubin GS. Feasibility of Macular Integrity Assessment (MAIA) microperimetry in children: Sensitivity, reliability, and fixation stability in healthy observers. Invest Ophthalmol Vis Sci. 2016 Nov 1;57(14):6349-6359. doi: 10.1167/iovs.16-20037.
17. Han S, Baek SH, Kim US. Comparison of three visual field tests in children: frequency doubling test, 24-2 and 30-2 SITA perimetry. Semin Ophthalmol. 2017;32(5):647650. doi: 10.3109/08820538.2016.1157611.
18. Patel DE, Cumberland PM, Walters BC, et al. Comparison of quality and output of different optimal perimetric testing approaches in children with glaucoma. JAMA Ophthalmol. 2018 Feb 1;136(2):155-161. doi: 10.1001/jamaophthalmol.2017.5898.
19. Gupta V, Kong GXY, Singh A, et al. measuring visual fields in children with glaucoma using a portable tablet. Transl Vis Sci Technol. 2014 May 1;13(5):10. doi: 10.1167/ tvst.13.5.10.