mieducation
Assessing Corneal Epithelial Cells with In Vivo Confocal Microscopy
Impaired central corneal epithelial cell density can have clinical implications including reduced corneal integrity, delayed healing, increased risk of infections, visual disturbances, and contact lens intolerance. In this article, Dr Pradipta Bharracharyya and Associate Professors Katie Edwards and Katrina Schmid describe the appearance of the epithelial cells with in vivo confocal microscopy (IVCM ), their regional density, and factors that affect this.
WRITERS Dr Pradipta Bhattacharyya and Associate Professors Katie Edwards and Katrina Schmid
LEARNING OBJECTIVES
On completion of this CPD activity, participants should be able to:
1. Explain the functional importance of the corneal epithelium,
2. Understand how in vivo confocal microscopy (IVCM) images can be analysed to give cell density,
3. Describe how the epithelial cell density varies across the cornea, and
4. Understand the implications of reduced cell density.
The cornea is the most anterior layer of the eye; its refractive power and clarity is critical for vision. The surface layer is the corneal epithelium, which is ~50μm thick, i.e., ~10% of the total corneal thickness. There are three main cell types: the superficial squamous stratified epithelial cells, the intermediate wing cells, and the inner basal cells.1
New cells originate from mitotic activity within the limbal stem cell niche (Figure 1), located within the palisades of Vogt. These new limbal basal cells move both centripetally and superficially within the cornea. Lemp and Mathers in 19892 described this based on observations of healing of corneal epithelial abrasions and the vortex patterns of corneal verticillate in Fabry’s disease and toxic keratopathies. They stated that “it is clear that there is evidence that epithelial cell regeneration is largely centred in the peripheral cornea and limbus, that there is a general, centripetal movement from the periphery to the centre of the cornea, and that this pattern is operative in the normal renewal of epithelial cells in the cornea”. Animal work and modelling has since confirmed this.3
The basal stem cells are the only cell type in the corneal epithelium that are capable of mitosis. The intermediate wing cells and the superficial stratified epithelial cells are derived from terminal differentiation of the basal cells, which move anteriorly to populate more superficial cell layers.4,5 There is a precisely regulated balance between proliferation, differentiation, and shedding of epithelial cells.6 This process maintains the epithelium’s integrity and acts to heal epithelial defects. Most corneal abrasions re-epithelialise quickly, however in some eye conditions defects can become persistent (persistent epithelial defects or PEDs).
Loss of the limbal stems cells and/or their niche causes limbal stem cell deficiency (LSCD),7 and in this condition the conjunctival epithelium invades the cornea. Corneal opacity, pain, and loss of vision can occur. LSCD can be genetic, acquired, or idiopathic.
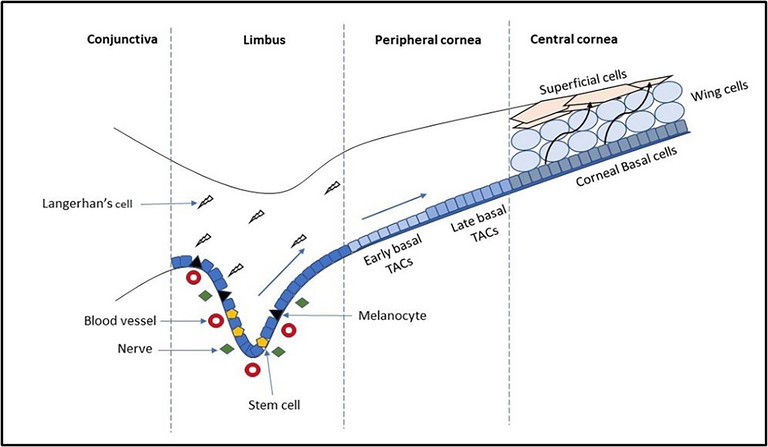
Figure 1. Schematic representation of the limbal stem cell niche. The corneoscleral limbus contains the palisades of Vogt. The limbal stem cells are in close contact with blood vessels, nerves, immune cells, and melanocytes. The stem cells divide to give rise to transient amplifying cells (TACs). The TACs then proliferate rapidly while migrating centripetally and form early and late basal TACs. After reaching the central corneal region, the basal TACs undergo terminal differentiation and give rise to post-mitotic cells that migrate apically to form the wing and superficial epithelial cell layers. Adapted from Li et al, 20078 by Pradipta Bhattacharya.
IMAGING OF THE CORNEAL EPITHELIAL CELLS
With the invention of IVCM in 1957, imaging of the corneal epithelial cells of the living human eyes, with image quality comparable to histological sections, became possible. The corneal and limbal epithelium, due to their unique formation (differentiated cells with neural innervation), relative transparency, avascularity, and thin structure, make these tissues amenable to imaging with this technique. The corneal epithelial cell layers and nerves that traverse them can be imaged and identified based on their appearance and depth within the cornea (Figure 2).9
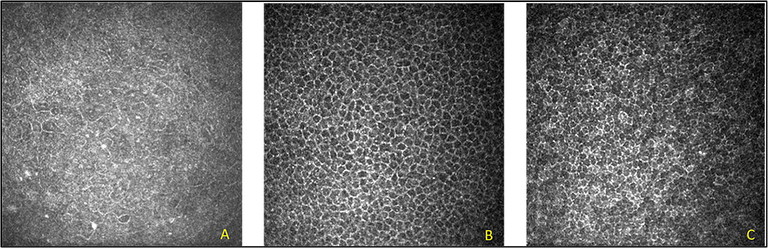
Figure 2. IVCM images of the normal cornea showing corneal epithelial cell structures observed at different focal depths. (A) superficial epithelial cells (focal depth: 10 µm), (B) wing cells (focal depth: 20–30 µm), (C) basal cells (focal depth: 30–40 µm).10
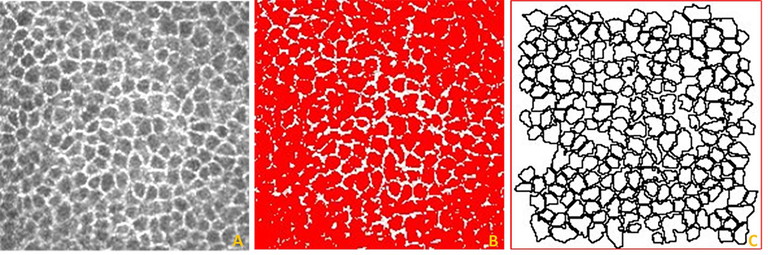
Figure 3. Identification and segmentation of corneal epithelial cells imaged using IVCM with the thresholding technique. (A) Volume scan images of the corneal epithelial cells (intermediate cells). (B) Use of image thresholding to identify the individual cells. (C) Individually segmented cells which can be analysed for their morphometric parameters. Image resolution: 96 dots per inch (DPI).10
The most superficial epithelial cells are particularly difficult to image (Figure 2A). They appear as hexagonal cells with bright cell borders with dark cell nuclei at a focal depth of approximately 10 µm from the corneal surface. The cell nucleus appears bright, and the dark perinuclear space is evident using an IVCM. The intermediate/ wing cells can be found at a focal depth of approximately 20–30 µm from the corneal surface. These cells have bright cell borders with dark cytoplasm (Figure 2B). The basal cells are the innermost last cellular layer of the corneal epithelium, located just anterior to Bowman’s membrane and can be imaged at a focal depth of 30–40 µm (Figure 2C). The basal cells have bright cell borders and dark cell bodies in which the nucleus is not visible. They also display homogeneous reflectivity of the cytoplasm, making it difficult to identify individual cell boundaries.10
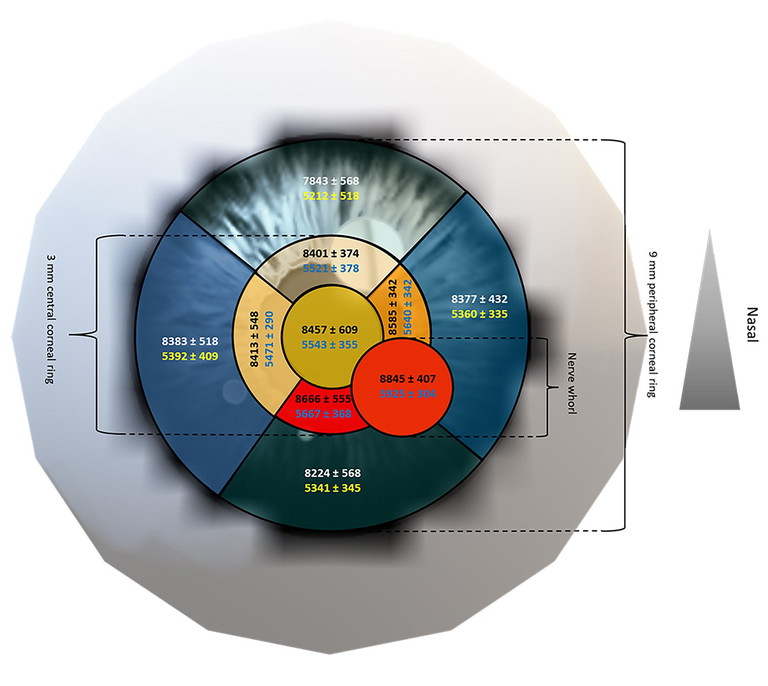
Figure 4. Regional variation in corneal basal and wing cell density (mean ± SD, cell/mm2 ). Legend: Basal cell (top number in black/white) and wing cell density (bottom number in dark blue/yellow). Representative image (not to scale); warmer colours indicate higher cell densities. 20
AUTOMATED IMAGE ANALYSIS
Manual image analysis of the corneal cells using freehand tools is time consuming and tedious. We investigated whether automated segmentation using Image J software (Figure 3) could be used to measure wing and basal cell density.10 We found that a manual thresholding technique, where the examiner controlled the threshold level, could be used to measure intermediate wing cells but not basal cell parameters. This is a partially automated system using free software that can assist in the quantification of these corneal cells.
CELL DENSITY ACROSS THE CORNEA
As it is easier to image the central cornea than the peripheral cornea using IVCM, most published studies describe central corneal epithelial cell morphology (mostly cell density and size),11-19 with very few studies of the peripheral cornea.14,15,18,19 While previously the highest cell density has been reported for the peripheral cornea, rather than the central cornea,4,14,18,19 we have shown that density is in fact highest at the central cornea and anterior to the corneal nerve whorl.20 Both the basal and wing cell density were highest anterior to the corneal nerve whorl and lowest in the superior peripheral cornea, i.e., 4.5 mm from the corneal apex (Figure 4). Locations closest to the position of the nerve whorl also had high cell density.
The peak basal cell density in young healthy adults was ~8900 cells/mm2 anterior to the whorl and was least ~7800 cells/mm2 in the superior cornea. Similarly, the maximum wing cell density of ~6000 cells/mm2 occurred anterior to the nerve whorl and the density was also least ~5200 cells/mm2 , superiorly. The nerve whorl, where the corneal nerves anastomose, is usually located, slightly inferior to the corneal apex and is an important physiologic as well as a topographic landmark.21,22 As it can be reliably located, it can be useful in tracking the progression of corneal disease.4 The ratio of the basal to wing cell densities was approximately 1.6 at all locations.
Our data supports the view that the cells are moving towards an inferior nasal position, anterior to the nerve whorl and not the corneal apex. This was suggested by Lemp and Mathers (1989)2 based on changes in cell size of corneal histological sections across the cornea. They reported that the central cornea, the superior peripheral cornea, and the inferior peripheral cornea have had statistically significant differences in cell area and perimeter. They suggested that the epithelial cell movement from the periphery to the centre of the cornea was non-uniform, with cells likely moving towards a point inferior to the corneal apex, which we now recognise as the position anterior to the corneal nerve whorl. It has recently been demonstrated in mice that corneal epithelial cell migratory tracks are aligned with adjacent nerves fibres, providing the most convincing evidence to date that these cell types migrate in tandem.23
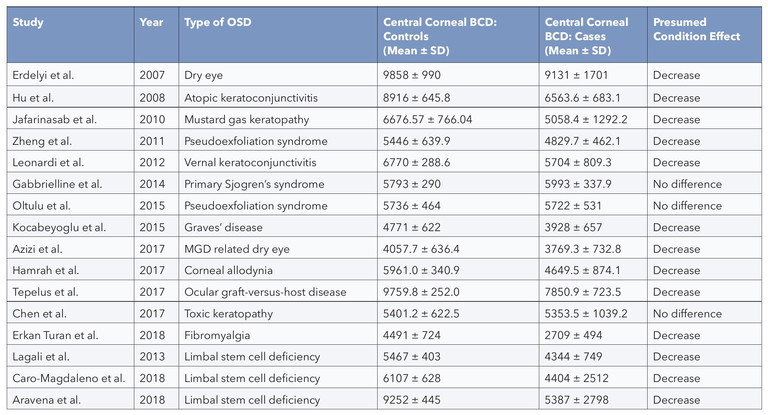
Table 1. Studies reporting the central corneal BCD in ocular surface disease (OSD) and limbal stem cell deficiency (LSCD). 28
EPITHELIAL CELL DENSITY IN DISEASE
Knowing the range of corneal cell density in healthy eyes is important in assessing the effect of disease and environmental impacts. Unanswered questions include what degree of cell reduction can be physiologically tolerated or compensated for and what is the threshold reduction at which function is impaired?
More about this is known for the corneal endothelium where typical adult cell densities are 2,400–3,200 cells/mm² and decrease with age.24 Endothelial cell density can fall below the threshold level needed to maintain corneal dehydration, resulting in corneal oedema. If extreme, it can cause a bullous keratopathy. This threshold of endothelial cell density varies considerably among individuals but is typically in the range of 500–1,000 cells/mm².
There are conditions known to affect the corneal epithelium, for example Lisch epithelial corneal dystrophy (LECD), which is a bandshaped and whorled microcystic dystrophy.25 However, although the epithelium can be imaged using IVCM, the corneal basal cell epithelial opacities mean that reliable cell counts are not possible. In conditions like these, it is thought that the affected epithelial basal cells originate from abnormal limbal stem cells.
Ocular conditions observed to affect central corneal epithelial cell density range from diseases directly affecting the ocular surface such as dry eyes, various allergies, and auto immune diseases, to ocular trauma that causes destruction to the limbal stem cell niche (Table 1). There are also reports of observed reduction in epithelial cell density due to contact lens wear.
In our 2022 review and meta-analysis of IVCM studies measuring central corneal basal cell density in ocular surface disease (OSD) and limbal stem cell deficiency (LSCD), the collated evidence showed there was a reduction in cell density in both conditions. However, as can be seen in Table 1, although all studies of LSCD showed this reduction, some OSD studies report no effect on basal cell density. This most likely reflects the difference in severity of the conditions and perhaps the degree to which the basal cell niche has been compromised.
“A reduction in central corneal epithelial cell density can have several clinical implications”
There was also large variation in the measured central basal cell density in healthy control eyes, which ranged from 4,000–9,900 cells/mm2 (Table 1). This variation may be due to undiagnosed ocular surface conditions, varying imaging techniques, and analysis methods. It is unlikely to be due to age differences of control participants. In a study of 80 healthy individuals, ranging in age from ~10 to 79 years, Zheng and co-workers report a small decrease in peripheral but not central basal epithelial cell density with age.19 However, this difference of about 6% occurred between the youngest participants aged ≤ 19 years and the other age groups; there was no difference in density between the ages of 20 to 79. In this respect, the corneal epithelium and endothelium differ, as it is well known that the corneal endothelial cell density decreases with age.26 This age-related reduction in endothelial, but not epithelial cells, is not surprising given the differences in the mitotic and replenishment characteristics of the two cell types.
CELL DENSITY REDUCTION
There is very little information on why corneal epithelial cell density is reduced in specific conditions. However, if the health and number of limbal stem cells are affected, this will affect cell density of all the corneal epithelial cell types. In this situation, there will be a reduced number of basal epithelial cells produced and as these differentiate into the intermediate wing and superficial squamous cells, then it follows that the cell density of these cell types will also be affected.
In limbal stem cell disease, the basal cell density – both centrally and at the limbus – is significantly reduced.27 When there are not enough functional stem cells to maintain the required supply of new corneal basal cells, then cell renewal is diminished. The precisely regulated process of proliferation, differentiation, and shedding is compromised. Ultimately, epithelial defects are not healed, and the normal transparent corneal epithelial surface is not maintained.
CLINICAL IMPLICATIONS AND CONCLUSION
A reduction in central corneal epithelial cell density can have several clinical implications:
1. Impaired corneal integrity: The corneal epithelium acts as a protective barrier against the external environment. A reduction in central corneal epithelial cell density can lead to impaired corneal integrity, making the cornea more susceptible to damage from external factors such as trauma or infection.
2. Delayed corneal healing: The corneal epithelium is responsible for the regeneration of corneal tissue. A reduction in central corneal epithelial cell density can lead to delayed corneal healing in case of injury or surgery.
3. Increased risk of infections: The corneal epithelium plays a crucial role in preventing infections by providing a physical barrier against pathogens. A reduction in central corneal epithelial cell density can increase the risk of infections, especially in individuals wearing contact lenses.
4. Visual disturbances: The corneal epithelium is responsible for maintaining the optical properties of the cornea. A reduction in central corneal epithelial cell density can cause visual disturbances such as haloes, glare, and blurred vision.
5. Contact lens intolerance: Individuals with reduced central corneal epithelial cell density may experience discomfort and intolerance while wearing contact lenses due to reduced oxygen permeability and increased risk of infections.
In summary, reduced central corneal epithelial cell density can have several clinical implications, including impaired corneal integrity, delayed healing, increased risk of infections, visual disturbances, and contact lens intolerance. It is important to monitor the central corneal epithelial cell density and take appropriate measures to prevent or manage conditions that alter the stem cell niche and alter proliferation, differentiation, and/or shedding of epithelial cells.
To earn your CPD hours from this article visit: mieducation.com/assessing-corneal-epithelialcells-with-in-vivo-confocal-microscopy.
References
1. Eghrari AO, Riazuddin SA, Gottsch JD. Overview of the cornea: structure, function, and development. Prog Mol Biol Transl Sci. 2015;134:7-23. doi: 10.1016/bs.pmbts.2015.04.001.
2. Lemp MA, Mathers WD, Corneal epithelial cell movement in humans. Eye (Lond). 1989;3 (Pt 4):438-45. doi: 10.1038/eye.1989.65.
3. Lobo E, Delic N, Richardson A, et al. Self-organized centripetal movement of corneal epithelium in the absence of external cues. Nat Commun. 2016;7:12388. doi: 10.1038/ncomms12388.
4. Krachmer JH, Mannis MJ, Holland EJ. Cornea: fundamentals, diagnosis and management. Third Edition, Elsevier Inc. 2011.
5. Mort RL, Douvaras P, Morley SD, et al. Stem cells and corneal epithelial maintenance: insights from the mouse and other animal models. Results Probl Cell Differ. 2012;55:357-94. doi: 10.1007/978-3-642-30406-4_19.
6. Sridhar MS. Anatomy of cornea and ocular surface. Indian J Ophthalmol. 2018;66:190-194. doi: 10.4103/ijo.IJO_646_17.
7. Ahmad S. Concise review: limbal stem cell deficiency, dysfunction, and distress. Stem Cells Transl Med. 2012;1(2):110-5. doi: 10.5966/sctm.2011-0037.
8. Li W, Hayashida Y, Chen YT, Tseng SC. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007 Jan;17(1):26-36. doi: 10.1038/sj.cr.7310137.
9. Jalbert I, Stapleton F, Papas E, et al. In vivo confocal microscopy of the human cornea. Br J Ophthalmol. 2003 Feb;87(2):225-36. doi: 10.1136/bjo.87.2.225.
10. Bhattacharya P, Edwards K, Schmid KL. Segmentation methods and morphometry of confocal microscopy imaged corneal epithelial cells. Cont Lens Anterior Eye. 2022;45(6):101720. doi: 10.1016/j.clae.2022.101720.
11. Mustonen RK, McDonald MB, Srivannaboon S, et al. Normal human corneal cell populations evaluated by in vivo scanning slit confocal microscopy. Cornea 1998;17:485-92. doi: 10.1097/00003226-199809000-00005.
12. Harrison DA, Joos C, Ambrósio R Jr. Morphology of corneal basal epithelial cells by in vivo slit-scanning confocal microscopy. Cornea 2003;22:246-8. doi: 10.1097/00003226-200304000-00013.
13. Vanathi M, Tandon R, Sharma N, et al. In-vivo slit scanning confocal microscopy of normal corneas in Indian eyes. Indian J Ophthalmol. 2003;51:225-30. PMID: 14601847.
14. Guthoff RF, Baudouin C, Stave J, Atlas of confocal laser scanning in-vivo microscopy in ophthalmology. Springer 2006.
15. Patel DV, Sherwin T, McGhee CN. Laser scanning in vivo confocal microscopy of the normal human corneoscleral limbus. Invest Ophthalmol Vis Sci. 2006;47:2823-7. doi: 10.1167/iovs.05-1492.
16. Niederer RL, Perumal D, Sherwin T, McGhee CN. Age-related differences in the normal human cornea: a laser scanning in vivo confocal microscopy study. Br J Ophthalmol. 2007;91:1165-9. doi: 10.1136/bjo.2006.112656.
17. Beuerman RW, Laird JA, Kaufman SC, Kaufman HE. Quantification of real-time confocal images of the human cornea. J Neurosci Methods. 1994;54:197-203. doi: 10.1016/0165-0270(94)90193-7.
18. Eckard A, Stave J, Guthoff RF. In vivo investigations of the corneal epithelium with the confocal rostock laser scanning microscope (RLSM). Cornea 2006;25:127-31. doi: 10.1097/01.ico.0000170694.90455.f7.
19. Zheng T, Le Q, Hong J, Xu J. Comparison of human corneal cell density by age and corneal location: an in vivo confocal microscopy study. BMC Ophthalmol. 2016;16:109. doi: 10.1186/s12886-016-0290-5.
20. Bhattacharya P, Edwards K, Schmid KL. Regional variations in corneal epithelial cell density and morphology assessed using In vivo confocal microscopy. Eye Contact Lens. 2024 Apr 1;50(4):163-170. doi: 10.1097/ICL.0000000000001067.
21. Petropoulos IN, Ferdousi M, Marshall A, et al. The inferior whorl for detecting diabetic peripheral neuropathy using corneal confocal microscopy. Invest Ophthalmol Vis Sci. 2015;56:2498-504. doi: 10.1167/iovs.14-15919.
22. Kalteniece A, Ferdousi M, Petropoulos I, et al. Greater corneal nerve loss at the inferior whorl is related to the presence of diabetic neuropathy and painful diabetic neuropathy. Sci Rep 2018;8:3283. doi: 10.1038/s41598-018-21643-z.
23. Tuck H, Park M, Carnell M, et al. Neuronal-epithelial cell alignment: A determinant of health and disease status of the cornea. The Ocular Surface. 2021; 21: 257-270. doi: 10.1016/j.jtos.2021.03.007.
24. Wilson RS, Roper-Hall MJ, Effect of age on the endothelial cell count in the normal eye. B J Ophthalmol. 1982;66:513-515. doi: 10.1136/bjo.66.8.513.
25. Chou C-Y, Lockington D, A corneal tulip? Assessing corneal opacities in Lisch corneal epithelial dystrophy. Clin Exp Optom. 2018;101: 596-598. doi: 10.1111/cxo.12555.
26. Sanchis-Gimeno JA, Lleó-Pérez A, Alonso L, et al. Corneal endothelial cell density decreases with age in emmetropic eyes. Histol Histopathol. 2005;20(2):423-7. doi: 10.14670/HH-20.423.
27. Chan EH, Chen L, Rao JY, et al. Limbal basal cell density decreases in limbal stem cell deficiency. Am J Ophthalmol. 2015;160(4):678-84.e4. doi: 10.1016/j.ajo.2015.06.026.
28. Bhattacharya P, Edwards K, Harkin D, Schmid KL, Central corneal basal cell density and nerve parameters in ocular surface disease and limbal stem cell deficiency: a review and meta-analysis. Br J Ophthalmol. 2020 Dec;104(12):1633-1639. doi: 10.1136/bjophthalmol-2019-315231.

Dr Pradipta Bhattacharya is a faculty member at Dehradun Institute of Technology University, India. He completed his Postdoctoral Research Fellowship at the University of Houston in 2024. He completed his PhD in 2021 from Optometry and Vision Sciences, Queensland University of Technology (QUT), under the guidance of Associate Professor Katrina Schmid, Associate Professor Katie Edwards, and Professor Damien Harkin. His doctoral research focussed on The Corneal Epithelium in Health and Disease. This article builds upon key findings from his research thesis.

Associate Professor Karie Edwards is an academic in optometry and vision science at QUT. She leads the Anterior Eye Laboratory at QUT, where her research interests include cellular level imaging of the anterior eye with confocal microscopy, investigating the anterior eye as a marker of systemic disease, and dry eye. Her teaching areas include anatomy, physiology, and diseases of the anterior eye.

Associate Professor Katrina Schmid is an academic in optometry and vision science at QUT. She is an experienced researcher, clinical optometrist, and educator, with extensive expertise in ocular surface disease and treatment. She is a Senior Fellow of the Higher Education Academy; this is an internationally recognised indicator of experience and expertise in learning and teaching in higher education. Her teaching expertise includes ocular anatomy and ocular pharmacology. Her research interests include assessment of the ocular surface, including meibomian gland dysfunction, and dry eye and its treatment.