miophthalmology
Diabetes and Diabetic Retinopathy: Shifting Paradigms in Treatment
WRITERS Dr Brighu Swamy and Dr Dov Hersh
The management of diabetic eye disease is undergoing a paradigm shift. New imaging modalities could potentially change the classification of diabetic retinopathy, and new options for treatment are on the horizon.
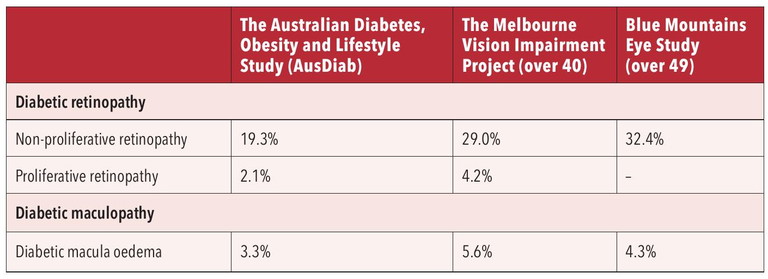
Table 1. Australian prevalence of diabetic retinopathy.
Diabetes is a chronic condition where the body cannot produce enough insulin, a hormone essential for converting glucose into energy. If poorly managed, diabetes can lead to vision-threatening eye disease in the form of diabetic macula oedema (DMO) and diabetic retinopathy (DR). Additional health complications include heart attack, stroke, and the need for limb amputation.
THE PREVALENCE OF DIABETES
Diabetes prevalence has slowly increased over the past 20 years, from 3.3% in 2001 to 5.3% in 2022. Like other chronic conditions, diabetes prevalence increases with age; from 1.0% for people aged 0–44 years, to 18.7% for people aged 75 years and over.1 A recently published article in Diabetes Research and Clinical Practice highlights a much larger and more diverse population living with diabetes, particularly older Australians, people in remote areas, and culturally and linguistically diverse communities. This article also highlights that Australian diabetes prevalence may be 35% higher than we believe.2
The Australian Bureau of Statistics highlights the demographics of people living with diabetes.1 In 2022, people were more likely to have diabetes if they were:
• Born overseas (6.7% compared to 4.7% of people born in Australia),
• Living in areas of most disadvantage (8.6% compared to 3.1% for those living in areas of least disadvantage),
• Living with disability (10.8% compared to 2.8% of people with no disability), and/or
• Living alone (11.3%).
Globally, more than 100 million individuals are living with DR, and DR is a leading cause of blindness and visual impairment, especially among the working-age adult population.3 Prevalence of DMO is projected to increase by about 25%, to about 24 million individuals by 2030.4
The Australian prevalence of diabetic retinopathy is summarised in Table 1.
EVOLVING TREATMENTS FOR TYPE 2 DIABETES
The treatment guidelines for type 2 diabetes mellitus (T2 DM) have changed significantly over the past 10 years. The 2018 American Diabetes Association guidelines for the treatment of T2 DM recommended glycaemic monotherapy as first line treatment. If the HbA1c was not optimal, a second drug would be introduced.
Sodium glucose cotransporter 2 inhibitors (SGLT2i) e.g. empagliflozin and dapagliflozin, and glucagon-like peptide-1 receptor agonists (GLP-1 RA) e.g. semaglutide and dulaglutide, have changed the treatment paradigm. These drugs have proven cardiorenal benefits while also reducing blood sugar levels, weight, and blood pressure. Current Australian guidelines recommend either of these drugs for patients with diabetes and concomitant cardiovascular disease, cardiovascular risk factors, or kidney disease.
This has implications for timing of diabetic retinopathy screening. Rapid increase in glucose control is associated with worsening DR. This has been known since the landmark Diabetes Control and Complications Trial (DCCT) Research Group in 1993.5

Dr Brighu Swamy

Dr Dov Hersh
“Retinal thinning is progressive over time and can precede the development of clinically visible DR lesions”
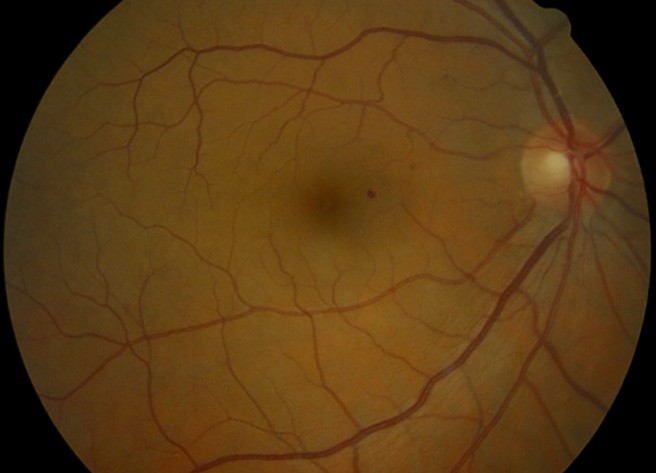
Figure 1. Mild NPDR with microaneurysm.
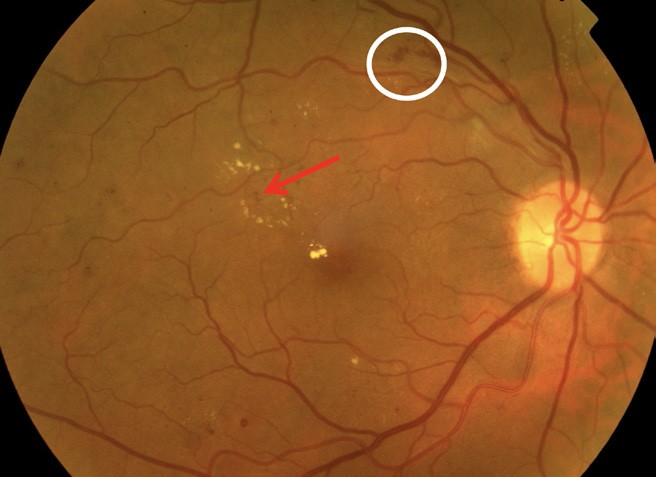
Figure 2. White circle: flame haemorrhage; red arrow: microaneurysm.
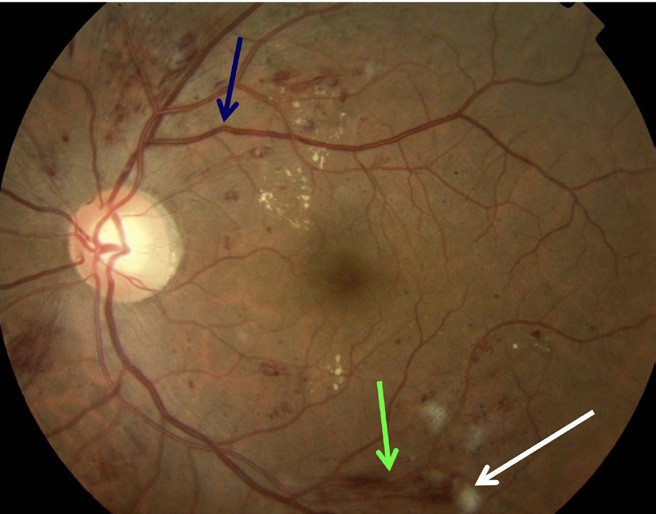
Figure 3. Blue arrow: early venous beading; green arrow: flame haemorrhage; white arrow: cotton wool spot.
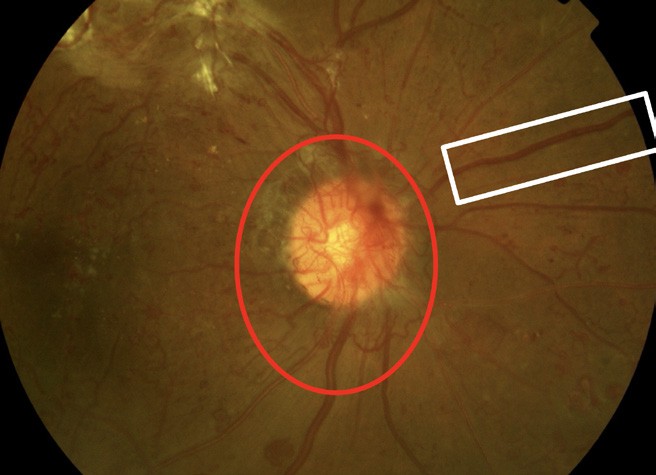
Figure 4. Red circle: optic disc new vessels; white rectangle: venous beading.
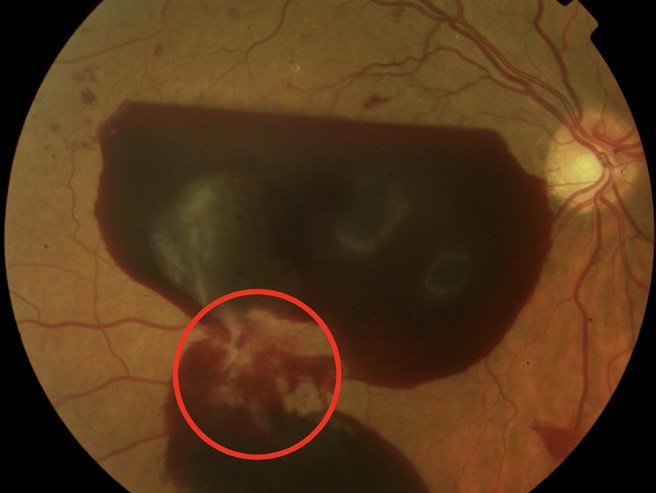
Figure 5. Retrohyaloid haemorrhage. Red circle: new vessels elsewhere with fibrosis.
In the SUSTAIN 6 trial, the semaglutide group had a 3% risk of DR complication vs 1.8% in the placebo group (hazard ratio 1.76).6 However, more recent studies suggest no worsening of diabetic retinopathy in those taking the GLP-1agonist drugs. Further GLP-1 agonists may confer neuroprotective and anti-inflammatory effects by modulating immune cell signalling and reducing pro-inflammatory cytokines.
For eye care professionals, this means a shorter interval for retinal screening in patients who have commenced GLP-1 receptor agonists or SGLT2i inhibitors. There is no need for routine eye screening for those who do not have diabetes and are taking these medications for weight loss.
CLASSIFICATION OF DIABETIC RETINOPATHY
Diabetic retinopathy is an umbrella term that covers all complications of diabetes that affect the retina. This includes nonproliferative diabetic retinopathy (NPDR), proliferative diabetic retinopathy (PDR), and maculopathy. There are two forms of diabetic maculopathy: diabetic macular oedema and diabetic macular ischaemia. Clinically visible retinal lesions associated with DR, such as microaneurysms, haemorrhages, and hard exudates, are primarily the result of retinal microvascular damage. Consequently, the focus on DR pathophysiology, diagnosis, and assessment has traditionally always centred around the vascular aspect of the disease.7
The current classification of diabetic retinopathy is based on the Early Treatment Diabetic Retinopathy Study (ETDRS).8 The ETDRS classification is still used for research and clinical trials today, but its widespread clinical application is limited by its complexity. In everyday clinical practice, the International Clinical Diabetic Retinopathy (ICDR) Severity Scale, which in essence is a simplified ETDRS system, is currently the most used classification system worldwide.9
Non-proliferative diabetic retinopathy is an early stage of the disease with fewer symptoms. NPDR is classified as mild, moderate, or severe (Figures 1–3).
NPDR can progress to proliferative diabetic retinopathy. Proliferative diabetic retinopathy refers to abnormal blood vessels that grow in the optic nerve, in the retina, or both, which can lead to vitreous haemorrhage (Figures 4 and 5). It can also cause scarring that can, in turn, lead to tractional retinal detachment and central and peripheral vision loss.
With the availability of better structural retinal imaging modalities and functional assessments, evidence has accumulated over the years of significant retinal neural dysfunction as well, which occurs together with, or in some cases precedes, the development of vascular abnormalities. These structural and functional changes have collectively been termed diabetic retinal neurodegeneration (DRN).7 Optical coherence tomography (OCT) studies have shown that patients with diabetes demonstrate significant thinning of the inner retinal layers, including the retinal nerve fibre layer (RNFL), and ganglion cell layer (GCL). Retinal thinning is progressive over time and can precede the development of clinically visible DR lesions. Functional assessments in diabetes reveal reductions in contrast sensitivity, visual field defects, electrophysiologic deficits, and impaired pupillary responses.
“Proliferative diabetic retinopathy refers to abnormal blood vessels that grow in the optic nerve”
Widefield and, in particular, ultra-widefield (UWF) retinal imaging has significantly advanced the detection, classification, and management of diabetic retinopathy. Traditional seven-field, 30° fundus photography, long considered the gold standard, covers only about 30% of the retinal surface, which can miss peripheral lesions that are now known to influence DR progression.10
In contrast, UWF imaging captures up to 200° of the retina in a single image, allowing clinicians to assess a much broader area without the need for multiple images or pupil dilation. Studies have demonstrated that UWF systems like Optos can detect peripheral lesions associated with a higher risk of DR progression – information often missed in conventional imaging.11 Assessment of the retinal peripheries with UWF images in DR has significant prognostic and management implications. For one, inclusion of the peripheries in UWF images results in a greater DR severity level in 10–19% of eyes.7
UWF fluorescein angiography has improved the detection and monitoring of nonperfusion areas and neovascularisation in DR, which are key factors in disease classification and treatment decisions.12
At present, the ideal modality for peripheral assessment in DR remains unclear. Nevertheless, it is evident that as we better define the role of the retinal peripheries, UWF imaging platforms will play an important role in DR assessment and management over the next decade.7
DIABETIC MACULOPATHY
At any stage of retinopathy, people may also develop DMO. This causes fluid to gather in the macula and can lead to loss of central vision (Figures 6 and 7).
OCT-angiography (OCT-A) will redefine how we classify diabetic maculopathy in the years ahead. OCT-A has emerged as a powerful, non-invasive imaging modality that detects subtle microvascular changes in diabetic eyes, often before clinical signs of diabetic retinopathy are visible. Table 2 is a summary of OCT-A findings in the various stages of diabetic retinopathy.
The first changes seen on OCT-A in patients with diabetic maculopathy are vascular remodelling, bordering the foveal avascular zone (FAZ), followed by tortuosity, narrowing of capillary lumens, dilatation of capillary ends, and formation of intraretinal microvascular abnormalities (Figure 8). One of the current disadvantages of OCT-A when compared with fluorescein angiography (FA) in the evaluation of diabetic retinopathy, is the inability to visualise vascular permeability seen on FA as leakage. Addtionally, some microaneurysms may not be well-delineated, as OCT-A is limited by the principle of slowest detectable flow.
Extraretinal neovascularisation, the hallmark of PDR, can be easily visualised by modifying the en face OCT-A slab and segmenting the vitreoretinal interface (Figure 9). In contrast to FA, this technique allows the extent and structure of the neovascular network to be evaluated without cumbersome dye leakage that may obscure its details.
Macular ischemia, easily recognisable on FA by an enlarged FAZ, can also be seen on OCT-A. However, the main advantages of OCT-A are its ability to measure the FAZ quickly and repeatedly due to its non-invasive nature and its provision of quantitative parameters, such as vascular density, circularity index, and FAZ area among others. These parameters are useful in monitoring retinal health and disease progression. Multiple studies have demonstrated that changes in the FAZ, quantified by various parameters, correlate with disease burden.13 This information may help clinicians identify eyes that require closer monitoring, early treatment, or referral.
A recent review highlights the critical role of the intermediate capillary plexus, often overlooked in conventional evaluations, and suggests its disruption is an early marker for DR.14 Studies comparing various OCT-A devices have shown that subclinical microvascular changes can be detected, even in diabetic patients without apparent retinopathy. The major impact from OCT-Awill be realised when such OCT-A metrics are eventually linked to clinical outcomes of interest on longitudinal studies. It is likely that OCT-A will become a powerful, non-invasive prognostic tool for clinical assessment in DR.
In summary, with newer imaging modalities now common in clinical practice, it is likely that the older classification systems for diabetic retinopathy and maculopathy will need to change in the years ahead.
ARTIFICIAL INTELLIGENCE SCREENING
In recent years, artificial intelligence (AI) tools for screening DR have progressed from trials to clinical application in various healthcare systems internationally. AI models, particularly those based on convolutional neural networks (CNNs), have significantly improved both the reach and accuracy of early DR detection.
The AI system IDx-DR (Digital Diagnostics) was the first autonomous AI DR screening device approved by the United States Food and Drug Administration (US FDA) in 2018. As of 2025, IDx-DR has been deployed in more than 2,000 primary care clinics across the US, reducing referral delays by more than 60%.15 It operates without the need for ophthalmologist oversight and provides immediate, point-of-care diagnostic decisions, identifying more-thanmild DR (mtmDR) with 95% sensitivity and 91% specificity.16 Currently, three AI platforms have been approved by the FDA for DR screening: AEye-DS (AEYE Health); EyeArt AI screening system (Eyenuk); and LumineticsCore, formerly IDx-DR.
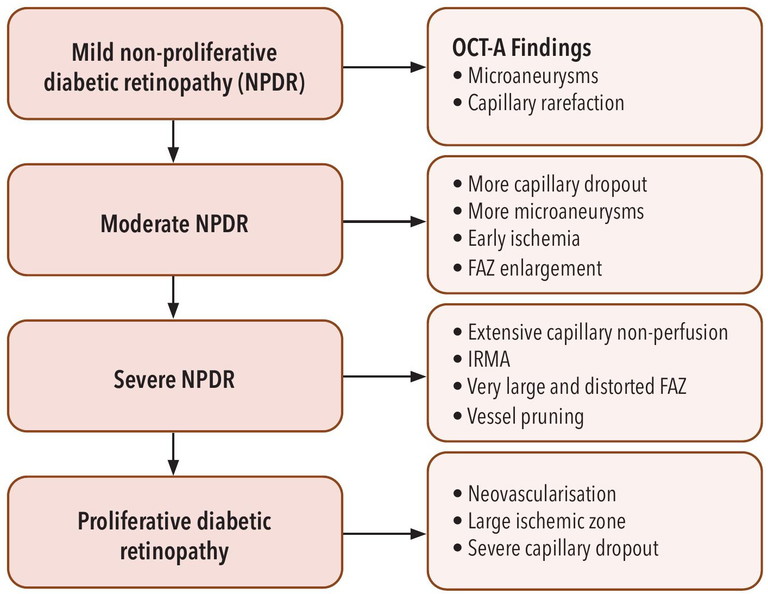
Table 2. OCT-A findings in the various stages of diabetic retinopathy.
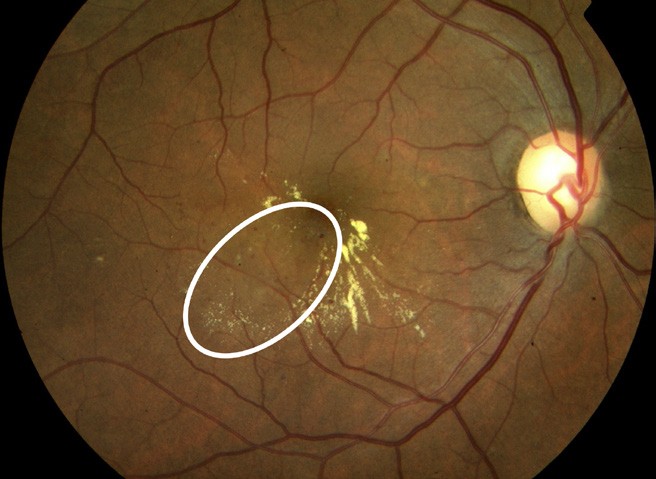
Figure 6. White eclipse: microaneurysms with hard exudates and retinal thickening.
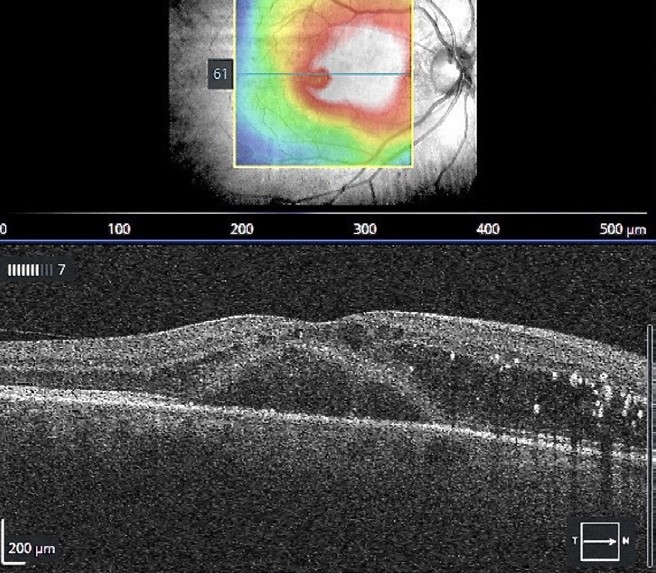
Figure 7. OCT of the macula showing centre involving macular oedema (CSMO).
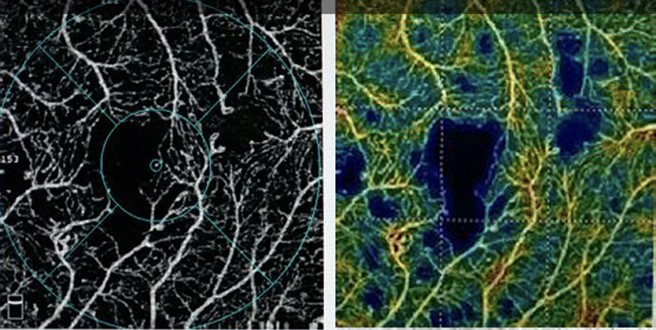
Figure 8. OCT-A with quantitative, colour-coded perfusion density mapping showing capillary drop out, microaneurysms, enlarged FAZ and decreased vascular density.
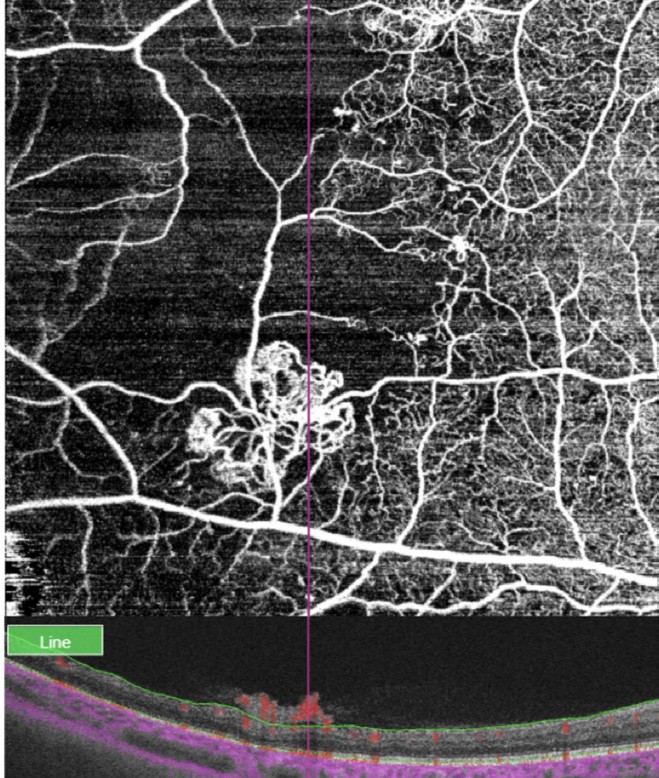
Figure 9. OCT and OCT-A of a patient with diabetes and severe PDR showing the anatomical location and blood flow of neovessels. By modifying the en face OCT-A slab and segmenting the vitreoretinal interface, the extra retinal neovascularisation can be easily visualised.
In India, Google Health’s AI model, developed in collaboration with Aravind Eye Hospital, has been integrated into more than 250 screening centres. Peer-reviewed results published in JAMA Ophthalmology showed the system achieving 97.3% sensitivity and 96.4% specificity for referable DR when tested across diverse patient demographics.17 The UK’s National Health Service (NHS) Diabetic Eye Screening Programme has also begun piloting AI tools such as EyeArt. In trials conducted with more than 100,000 patients, EyeArt demonstrated over 96% sensitivity for detecting referable DR,18 enabling faster triage and easing the workload of ophthalmic graders. AI is now recognised in multiple national guidelines (e.g., American Diabetes Association 2025 Standards of Care) as a viable first-line screening option for DR, especially in low-resource settings.
In the Australian context, the Therapeutic Goods Administration (TGA) granted clearance for autonomous DR screening devices under new digital health regulations, introduced in 2024, that focus on real-world validation, data privacy, and algorithmic transparency. In Western Australia, the Indigenous focussed Warlu AI system is being rolled out under the guidance of Professor Angus Turner and the Lions Eye Outback service.19
Despite significant advances, barriers for adoption of AI screening for DR include the need for representative datasets to ensure algorithmic performance across diverse populations. Currently, many existing models are trained on limited demographic groups, raising concerns about bias and reduced accuracy in underrepresented communities. Integration into existing healthcare workflows also requires substantial infrastructural support, including digital imaging capabilities, stafftraining, and interoperability with electronic medical records. Regulatory and medico-legal uncertainties – particularly around liability in cases of misdiagnosis – further complicate adoption. A system with a sensitivity of 95% will miss one in 20 patients needing referral for DR. Data privacy, informed consent, and maintaining transparency in decision-making algorithms are also ongoing ethical and operational considerations.
TREATMENT OF DIABETIC RETINOPATHY
Current treatment options for DR encompass both well-established and emerging approaches, aimed at halting or reversing vision loss due to diabetes-related retinal damage.
Pharmacologic Therapies Intravitreal anti-VEGF (vascular endothelial growth factor) injections are the frontline treatment for DMO, a major cause of vision loss in DR. These include agents such as ranibizumab, aflibercept, and bevacizumab, which have demonstrated significant efficacy in reducing retinal swelling and improving visual acuity.
New treatments that are more likely to have significant impact on the DR landscape over the next decade are those targeting new pathophysiologic pathways, and those that improve the durability of treatment effect. For example, faricimab is a bi-specific monoclonal antibody that provides dual inhibition of both the VEGF and the angiopoietin (Ang) and tyrosine kinase with immunoglobulinlike and epidermal growth factor homology (Tie) pathways. Inhibiting Ang-2 on top of VEGF-A is thought to provide a synergistic effect, with better vascular stability and reduction in vascular leakage.7 The recent Phase 3 YOSEMITE and RHINE clinical trials demonstrated non inferiority of faricimab compared to aflibercept for DMO, but with superior anatomic outcomes. More importantly, faricimab had a durable treatment effect, with more than 70% and 50% of eyes reaching dosing intervals of every 12 to 16 weeks, and 16 weeks respectively at one year.20 Other promising treatment strategies to provide increased durability and reduced treatment burden include high dose aflibercept (8 mg).21
Corticosteroids, such as dexamethasone implants, are also employed, particularly in patients unresponsive to anti-VEGF therapy, despite a higher risk of intraocular pressure elevation and cataract formation.
Laser Photocoagulation This older but still utilised treatment is effective in stabilising advanced proliferative DR. Panretinal photocoagulation (PRP) helps prevent neovascularisation by ablating peripheral retinal ischemia and thereby reducing VEGF production.
Vitreoretinal Surgery Pars plana vitrectomy is employed for cases of non-resolving vitreous haemorrhage or tractional retinal detachment. Improvements in surgical techniques have increased the success rate of restoring visual function in advanced cases.
Emerging Therapies and Innovations There is growing interest in extended-release intraocular implants and novel delivery systems aimed at reducing the treatment burden, as well as gene therapy with agents such as RGX-314 and ADVM-022 for long-term VEGF suppression.22,23 By providing more durable treatment effect, these approaches aim to address real unmet needs in DMO treatment, where high treatment burden, problems with compliance to therapy, and under-treatment, limit real world visual outcomes.24 These treatment approaches will play a major role in DMO management in the near future.
Preventive and Systemic Strategies Controlling blood glucose, blood pressure, and lipid levels remains essential in reducing the risk and progression of DR. Screening and early detection are key for effective intervention.
“In the years ahead we are going to see some rapid changes in how we classify, screen, and treat diabetic retinopathy”
Overall, diabetic retinopathy management has evolved to become a multi-modal approach combining pharmacotherapy, surgery, laser, and systemic care, with exciting prospects on the horizon for less invasive, more durable treatments.
CONCLUSION
In the years ahead we are going to see some rapid changes in how we classify, screen, and treat diabetic retinopathy. The global burden of diabetes is shifting to Asia, Africa, and Latin America. Artificial intelligence will be a powerful tool in this challenge to meet the demands that are increasing on eye care professionals around the globe.
To earn your CPD hours from this article, visit mieducation.com/diabetes-and-diabeticretinopathy-shifting-paradigms-in-treatment.
Dr Brighu Swamy BSc (Med) MBBS (Hons) MMed (Oph Sci Clin Epi) FRANZCO is a medical retina and macula subspecialist. He is a founding director at Sydney Eye Surgeons. His expertise is in the diagnosis, treatment, and care of patients with retinal conditions. Dr Swamy graduated in medicine from the University of New South Wales with Honours, before completing both a Master of Medicine in Ophthalmic Science and a Master of Medicine with merit in clinical epidemiology at the University of Sydney. He undertook ophthalmology training at the Sydney Eye Hospital and subsequently completed the medical retina and uveitis fellowship at the Moorfields Eye Hospital in London.
He is a clinical lecturer at the University of Sydney and a Visiting Medical Officer at Bankstown Hospital, where he is involved in teaching and supervising registrars.
Dr Dov Hersh BCom (IT) MBBS (Hons) MMed (Ophth) FRANZCO is a Sydney and NSW Central Coast-based ophthalmologist with sub-specialty expertise in diseases of the retina. After completing specialist ophthalmology qualifications at the Sydney Eye Hospital, Dr Hersh undertook rare dual international post-graduate fellowships at Bristol Eye Hospital and Moorfields Eye Hospital, London. Dr Hersh trained with pioneers in the field of medical retina and was appointed as an investigator in an array of multinational clinical trials, studying emerging treatments for retinal diseases and bringing this expertise to his patients. He remains a Clinical Associate Lecturer at the University of Sydney.
References available at mieducation.com.