mieducation
Surgical Management of Glaucoma in 2023: What’s New & What’s Still Working
There has been a rapid evolution of glaucoma surgery in the past two decades, leading to an ever-increasing array of treatment options for clinicians who treat glaucoma. Lowering intraocular pressure (IOP) to halt glaucoma progression remains the treatment aim for all surgeries. Innovations in glaucoma surgery aim to achieve this with less side effects and improved repeatability. Classical filtration surgery, however, is still the mainstay of surgical management owing to its efficacy in advanced glaucoma and long track record. Drs Aaron Wong and Ridia Lim look at developments in glaucoma surgery and review older surgical techniques that are still key in a glaucoma surgeon’s armamentarium.
WRITERS Dr Aaron Wong and Dr Ridia Lim
LEARNING OBJECTIVES
On completion of this CPD activity, participants should be able to:
1. Recognise the categories of aqueous outflow glaucoma surgeries available,
2. Understand the risks and benefits of both traditional and newer glaucoma surgeries, and
3. Recognise new developments in glaucoma surgical management.
To lower IOP, the majority of glaucoma surgeries aim to increase aqueous outflow from the anterior chamber. This can be broadly classified into: subconjunctival drainage, trabecular drainage, and suprachoroidal drainage. 1Afurther category of glaucoma surgery is also on the horizon however; implantable drug-eluting devices (Figure 1).
SUBCONJUNCTIVAL DRAINAGE
Trabeculectomy
Despite being first performed over 50 years ago, trabeculectomy remains the benchmark for bleb forming glaucoma surgery. Although many variations on the technique exist, all trabeculectomies require egress of aqueous through a sclerostomy and partial thickness scleral flap to form a subconjunctival bleb. Trabeculectomy techniques have evolved over time and most modern techniques involve the use of releasable or laserable flap sutures and antifibrotics, such as mitomycin C (MMC) and 5-fluorouracil (5FU).
The result is a surgery that is both very effective in lowering IOP but also modifiable in the post operative period. A multicentre analysis showed that at two years, 87% of patients were able to achieve a 20% reduction in IOP with medications and 80% met this criteria without medications.2Twenty year outcomes are available for trabeculectomy, with two studies finding 79% 3and 88% 4of patients are able to maintain an IOP <21mmHg with medications. Trabeculectomy does come with significant risk, however. Early complications, such as hyphaema (24.6%), shallow anterior chamber (23.9%), hypotony (24.3%), choroidal detachment (14.1%), bleb leak (17.6%), and endophthalmitis (0.4%) have been reported.5Late complications include: cataract (20.2%), vision loss of more than one Snellen line (18.8%), bleb encapsulation (3.4%), endophthalmitis (0.2%), and wipe out (5% of advanced glaucoma). 5
Deep Sclerectomy
Deep sclerectomy (DS) is a form of nonpenetrating glaucoma surgery that has been used for over 30 years. 6Although similar to a trabeculectomy, a DS involves removal of a partial thickness scleral flap and dissection up to, but not through, the Trabeculo-Descemet’s membrane (TDM). Compared with a trabeculectomy, DS has an additional site of resistance to aqueous outflow at the TDM (which can be penetrated post-operatively with laser goniopuncture) and allows flow into an intrascleral lake prior to a subconjunctival bleb. Clinically, it can be difficult to distinguish a DS from a trabeculectomy, except for the presence of an iridectomy or an ostium on gonioscopy, which confirms a trabeculectomy rather than a DS. A recent retrospective review found a similar reduction in IOP post trabeculectomy (38%) compared with DS (34%) at two years, however DS required fewer post-operative surgical interventions (13% compared to 30%) and fewer postoperative visits.7(Figure 2).
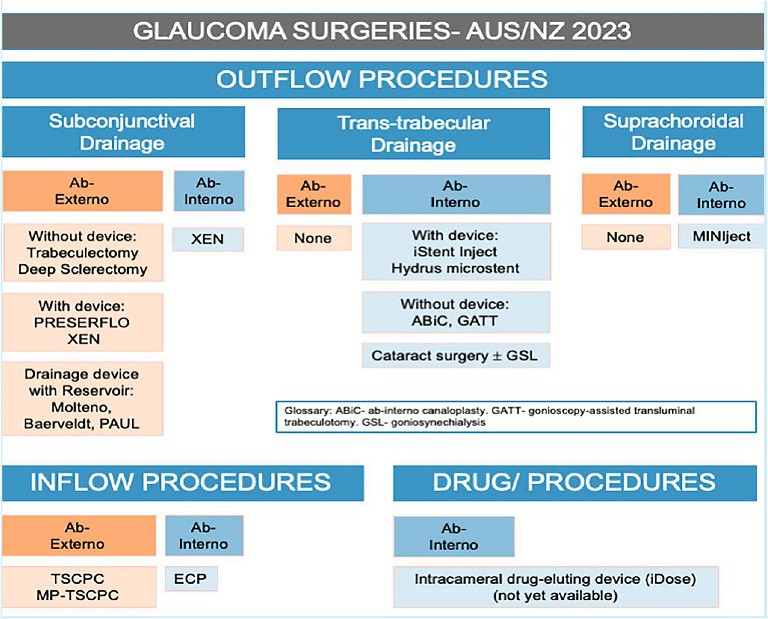
Figure 1. Glaucoma surgeries available in Australia and New Zealand.
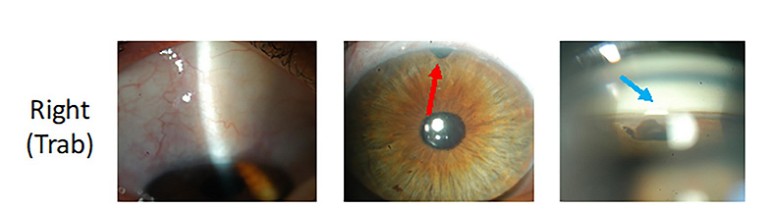
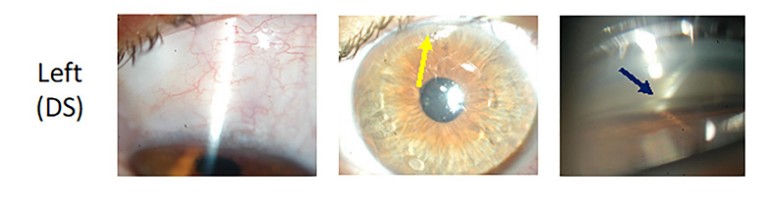
Figure 2. Appearance of a trabeculectomy (right) vs. deep sclerectomy (left) in the same patient. Despite similar bleb appearances, the trabeculectomy has an iridectomy (red arrow) whereas the DS does not (yellow arrow). Also, on gonioscopy the trabeculectomy has an ostium (light blue arrow) and the DS has a TDM (dark blue arrow).
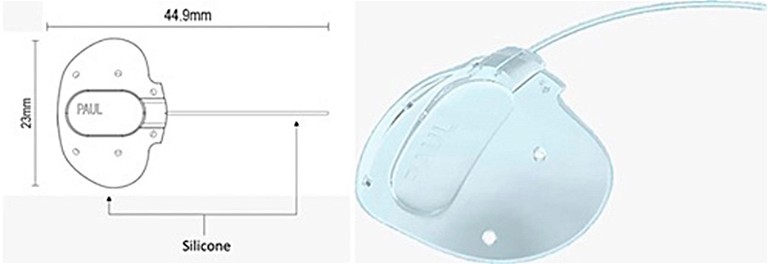
Figure 3. Paul glaucoma implant design (Advanced Ophthalmic Innovations).13
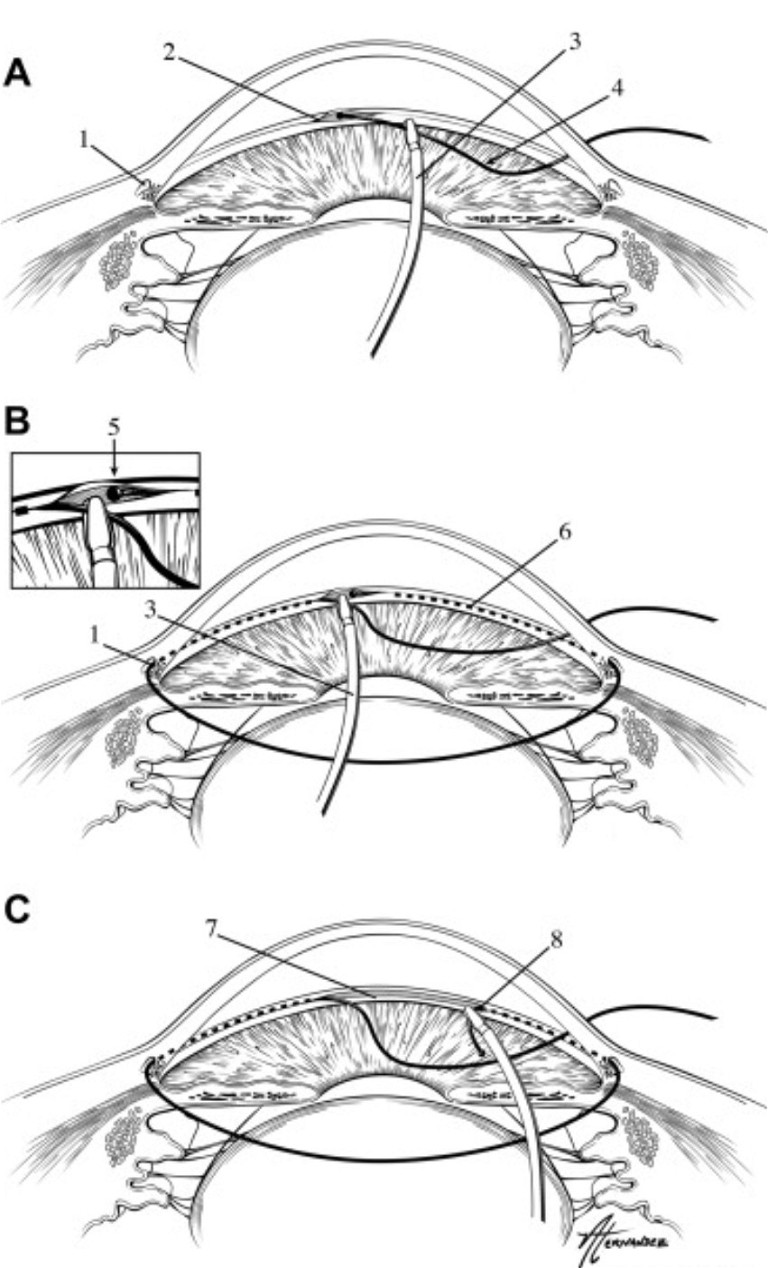
Figure 4. Gonioscopy-assisted transluminal trabeculotomy (GATT).18
Glaucoma Drainage Devices
Implantation of a glaucoma drainage device (GDD) tube to shunt aqueous from the anterior chamber to a subconjunctival plate was first performed by Molteno in 1969. 8 Over the following 50 years various tube shunts have been developed, including the valved Ahmed glaucoma implant (AGV, New World Medical) and non-valved Baerveldt implant (BGI 250 and 350, Johnson & Johnson Surgical Vision) as well as various iterations of the Molteno implant (Molteno single and double plate, Molteno3, Nova Eye Medical).
As well as having evidence of efficacy for 20 years, tubes have been shown to be effective in patients where trabeculectomy has either failed or would be at high risk of failure.9–11 In the Tube Versus Trabeculectomy study, patients with previous trabeculectomy or cataract surgery achieved a similar mean IOP at five years with tube surgery (14.4mmHg) compared with trabeculectomy (12.6mmHg) but with lower rates of reoperation (9% vs. 29%).12 Despite tube surgery having unique risks of erosion (5% at five years), persistent corneal oedema due to endothelial loss (16% at five years) and diplopia (6% at five years), overall the complication rate is lower than that of trabeculectomy. 1,12 Early hypotony is also a concern for non-valved tube surgery and is addressed by the use of a ligating suture, with or without an intraluminal stent suture, to occlude early flow while leaving the option to increase flow at a later stage.
A new tube shunt, the Paul glaucoma implant (PGI, Advanced Ophthalmic Innovations) was introduced six years ago. Compared with the BGI and Molteno3, the tube portion of the PGI has a smaller internal (127µm vs. 300µm) and external diameter (467µm vs. 630µm and 640µm), reducing the theoretical risk of hypotony, tube erosion, and endothelial cell damage.13 It has a comparable plate size (342.1mm2) compared to the BGI (250mm 2or 350mm2) and Molteno3 (185mm2 or 245mm2). Two year data shows the PGI lowered mean IOP to 13.9mmHg from 19.8mmHg preoperatively with a rate of hypotony requiring intervention of 8.9%.13 A group from the United Kingdom has reported a lower rate of hypotony (2%) with the use of an intraluminal stent and a similar IOP at one year of 13.3mmHg.14 Further studies will evaluate the long-term efficacy of this new GDD and potential long-term complications (Figure 3).
Preserflo MicroShunt
The Preserflo (Santen Inc) is a biocompatible tube made from polystyrene-blockisobutylene-block-styrene (SIBS). It is designed to be implanted ab externo in a tract made from the subconjunctival space into the anterior chamber. The tube is not attached to a plate and thus requires mitomycin C to form a long-term bleb, making it more akin to trabeculectomy surgery rather than GDD surgery. Theoretically, the Preserflo device’s length and internal diameter of 70µm is designed to restrict flow at a low IOP to avoid hypotony, however this relies on a normal rate of aqueous production and no flow around the tube. In a head-to-head comparison, Preserflo patients had a slightly higher IOP at two years compared to trabeculectomy (14.3mmHg vs. 11.1mmHg) but lower rates of hypotony requiring intervention (2.0% vs. 7.6%).15 Preserflo was only introduced in Australia in 2022 but as the global experience with Preserflo increases, surgeons are widening the range of glaucoma subtypes it is used for, as well as establishing standardised techniques for consistent results.16
“ as the global experience with Preserflo increases, surgeons are widening the range of glaucoma subtypes it is used for ”
Xen Gel Stent
Released in 2016, the Xen gel stent (Allergan) is a crosslinked porcine gelatin tube designed to implanted ab interno from the anterior chamber and drain into the subconjunctival space. As well as differing in material and implantation to the Preserflo microshunt, the Xen also has a smaller lumen size at 45µm and shorter length (6mm vs. 8.5mm). A recently published randomised control trial (RCT) comparing Xen and trabeculectomy found that both surgeries had a similar rate (62.1% vs. 68.2%) of achieving the study’s primary target (IOP reduction of ≥20%, without increasing medications, severe vision loss or clinical hypotony). At 12 months, the mean IOP in the trabeculectomy group was lower (11.8mmHg) compared to Xen (14.4mmHg).17Similar to previous studies comparing Xen and trabeculectomy however, there was a higher rate of post-operative needling and secondary operations required in the Xen group. Because of this, a variety of techniques for implantation have been trialled including ab externo implantation with or without conjunctival dissection, as well as sub-tenon and subconjunctival placement of the stent in an effort to minimise bleb fibrosis. 1There is still uncertainty over which is the best approach and now that more subconjunctival devices have come to the market, the use of the Xen has decreased.
TRABECULAR DRAINAGE
iStent Infinite
The iStent trabecular micro-bypass system has been available for almost 10 years and is the most widely implanted trabecular microbypass device worldwide. Over this time, further iterations have continued to improve the efficacy and safety of the device.
Following on from the current iStent inject W (Glaukos) system, the iStent infinite (not currently available in Australia) will deliver three, rather than two, stents and allow the surgeon infinite re-deployments to maximise the likelihood of successful implantation.19Studies using the first generation iStent G1 (Glaukos) have shown that increasing from two to three stents can provide a modest improvement in IOP lowering (37% total IOP lowering increasing to 43%) without a compromise in safety. 20Previous versions of the iStent device have shown efficacy in patients with mild-tomoderate glaucoma without previous glaucoma surgery, often in combination with cataract surgery. 20,21However, the iStent infinite has shown promise as a standalone procedure in a cohort of patients with either previous glaucoma surgery or maximal medical therapy. 19
Hydrus Microstent
First released in Australia in 2014, the Hydrus microstent (Alcon) is a nictinol stent designed for ab interno implantation through the trabecular meshwork (TM) into Schlemm’s canal. Its design allows it to not only create a TM bypass but also dilate Schlemm’s canal for three clock hours. The HORIZON RCT compared cataract surgery with Hydrus implantation to cataract surgery alone. After two years, 77% of combined cases showed a diurnal IOP reduction of ≥20% compared with 57% of those with cataract alone. 22 Recently published Australian data from the Fight Glaucoma Blindness (FGB!) registry showed Hydrus and iStent inject implantation combined with cataract surgery were also effective in reducing IOP (reduction of 3.1mmHg for iStent and 2.3mmHg for Hydrus) as well as reducing glaucoma medications (reduction of 1.0 medications for iStent and 0.5 medications for Hydrus) at two years, with no statistically significant differences between the two devices.23
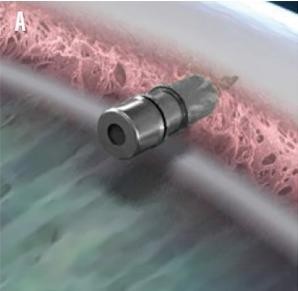
Figure 6. iDose TR.33
“ Studies using the first generation iStent G1 (Glaukos) have shown that increasing from two to three stents can provide a modest improvement in IOP lowering… without a compromise in safety ”
Gonioscopy-Assisted Transluminal Trabeculotomy
Gonioscopy-assisted transluminal trabeculotomy (GATT) was first described by Grover et al in 2014.24 Using an ab interno approach, a polypropylene suture or microcatheter is introduced via a goniotomy into Schlemm’s canal and advanced either 360° or 180° (hemi-GATT). The distal end of the suture or microcatheter is then retrieved and externalised, bisecting the TM. 24Grover et al. reported, at two years post GATT, that patients with primary open angle glaucoma (POAG) had an average IOP reduction of 37.3% and 49.8% in patients with secondary open angle glaucoma (OAG). 25 Transient hyphaema is a common complication of GATT, however, and has been reported in 39% of all cases (Figure 4).26
iTrack Ab-interno Canaloplasty
The iTrack advance (Nova Medical) is a new delivery system released in 2022 for the existing iTrack viscocanaloplasty device. The iTrack is a microcatheter with an illuminated fibreoptic tip that delivers viscoelastic directly to Schlemm’s canal to dilate the aqueous outflow system.
Previously, insertion of the microcatheter was performed in a similar fashion to the GATT procedure described above. The iTrack advance delivery system allows the procedure to be done using a single device in the eye. Three-year data on a small cohort of patients showed a decrease in mean IOP from 20.5mmHg at baseline to 13.3mmHg with no serious complications.27Both larger studies and comparative studies will be informative in the future.
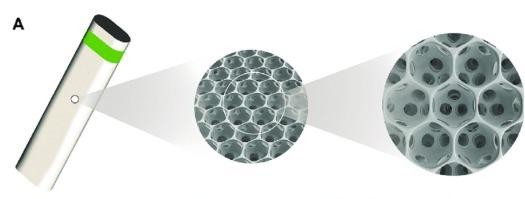
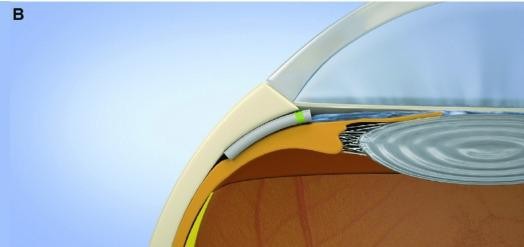
Figure 5. Miniject (iStar Medical) design and placement.30
SUPRACHOROIDAL DRAINAGE
Miniject
Recently approved by the Therapeutic Goods Administration (TGA) for use in Australia, the Miniject (iStar Medical) is a supraciliary device made of a soft, porous, flexible, and biocompatible material. 28 It is designed to enhance aqueous outflow into the suprachoroidal space, similar to the now withdrawn CyPass (Alcon) device. Two-year data is now available, showing a reduction in IOP in 25 patients from 23.2mmHg at baseline to 13.8mmHg (40.7% reduction) at two years. 28 As the Cypass was withdrawn due to concerns over endothelial cell loss, it was reassuring that the Miniject showed only a mild reduction in endothelial cell density (5% compared with matched controls). 29 However, larger and longer-term studies will be needed to further evaluate the device’s efficacy and long-term safety (Figure 5).30
IMPLANTABLE DRUG-ELUTING DEVICES
iDose
The iDose TR (Glaukos) is a 1.8mm x 0.5mm biocompatible titanium implant containing a proprietary formulation of travoprost. It is designed to be implanted into the TM. Three-year data shows a mean decrease in IOP of 8mmHg, and that a majority (68–70%) of patients were well controlled on less or the same topical medications as at implantation. In just over 100 patients receiving the implant, none experienced long-term hyperaemia or periorbital fat atrophy but a single patient did have iris pigmentation change.31The iDose implant has also been successfully exchanged for a new device in 32 patients who were part of the Phase 2b clinical trial. 32This is not yet available (Figure 6).
CONCLUSIONS
The surgical treatments available to glaucoma patients are expanding rapidly as we search for safer and more repeatable options to lower IOP, particularly for early to moderate glaucoma. Classical glaucoma filtering surgeries, such as trabeculectomy, deep sclerectomy, and aqueous tube shunts still have the advantages of long-term data and clinician familiarity, and hence they remain the treatment of choice in severe or complex glaucoma. Undoubtedly, in the future changes to this paradigm will bring about safer surgical options for our glaucoma patients.
Financial disclosures: The authors have been supported by Glaukos.
This article supplements a previous CPD module ‘MIGS in 2022: What Optometrists Need to Know’ with new developments in glaucoma surgery as well as looking back at older surgical techniques that are still key in a glaucoma surgeon’s armamentarium. That first CPD module is available at mieducation.com/pages/migs-in-2022-whatoptometrists-need-to-know.
To earn your CPD hours from this article visit mieducation.com/surgical-management-ofglaucoma-in-2023.
References
1. Lim, R., The surgical management of glaucoma: A review. Clinical & Experimental Ophthalmology. 2022 Mar;50(2):213–31.
2. Kirwan, J.F., Lockwood, A.J., Shah, P., et al., Trabeculectomy outcomes group audit study group. Trabeculectomy in the 21st century: a multicenter analysis. Ophthalmology. 2013 Dec 1;120(12):2532–9.
3. Bevin, T.H., Molteno, A.C., Herbison, P., Otago glaucoma surgery outcome study: long-term results of 841 trabeculectomies. Clinical & experimental ophthalmology. 2008 Nov;36(8):731–7.
4. Landers, J., Martin, K., Sarkies, N., et al., A twenty-year follow-up study of trabeculectomy: risk factors and outcomes. Ophthalmology. 2012 Apr 1;119(4):694–702.
5. Edmunds, B., Thompson, J.R., Salmon, J.F., Wormald, R.P., The national survey of trabeculectomy. III. Early and late complications. Eye. 2002 May;16(3):297–303.
6. Mendrinos, E., Mermoud, A., Shaarawy, T., Nonpenetrating glaucoma surgery. Survey of ophthalmology. 2008 Nov 1;53(6):592–630.
7. Dwivedi, R., Somerville, T., Cheeseman, R., et al., Deep sclerectomy and trabeculectomy augmented with Mitomycin C: 2-year post-operative outcomes. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2021 Jul;259:1965–74.
8. Molteno, A.C., New implant for drainage in glaucoma. Animal trial. The British Journal of Ophthalmology. 1969 Mar;53(3):161.
9. Molteno, A.C., Bevin, T.H., Herbison, P., Husni, M.A., Longterm results of primary trabeculectomies and Molteno implants for primary open-angle glaucoma. Archives of ophthalmology. 2011 Nov 10;129(11):1444–50.
10. Molteno, A.C., Bevin, T.H., Herbison, P., Houliston, M.J., Otago glaucoma surgery outcome study: long-term follow-up of cases of primary glaucoma with additional risk factors drained by Molteno implants. Ophthalmology. 2001 Dec 1;108(12):2193–200.
11. Dawson, E.F., Rosenberg, N.C., Meyer, A.M., et al., Comparison of outcomes of glaucoma drainage implant surgery with or without prior failed trabeculectomy. Journal of Glaucoma. 2021 Jul 10;30(7):585–95.
12. Gedde, S.J., Schiffman, J.C., Feuer, W.J., et al., Tube versus trabeculectomy study group. Treatment outcomes in the tube versus trabeculectomy (TVT) study after five years of follow-up. American Journal of Ophthalmology. 2012 May 1;153(5):789–803.
13. Tan, M.C., Choy, H.Y., Chang, V.K., et al., Two-year outcomes of the PAUL glaucoma implant for treatment of glaucoma. Journal of Glaucoma. 2022 Jun;31(6):449.
14. Vallabh, N.A., Mason, F., Yu, J.T., et al., Surgical technique, perioperative management and early outcome data of the PAUL glaucoma drainage device. Eye. 2022 Oct;36(10):1905–10.
15. Baker, N.D., Barnebey, H.S., Moster, M.R., et al., Abexterno microshunt versus trabeculectomy in primary open-angle glaucoma: one-year results from a 2-year randomized, multicenter study. Ophthalmology. 2021 Dec 1;128(12):1710–2.
16. Khawaja, A.P., Stalmans, I., Aptel, F., et al., Expert consensus on the use of the Preserflo microshunt device in the treatment of glaucoma: A modified delphi panel. Ophthalmology and Therapy. 2022 Oct;11(5):1743–66.
17. Sheybani, A., Vera, V., Grover, D.S., et al., Gel stent vs trabeculectomy: The randomized, multicenter, gold standard pathway study (GPS) of effectiveness and safety at 12 months: Gel stent vs trabeculectomy: A prospective randomized study. American Journal of Ophthalmology. 2023 Mar 25.
18. Schlenker, M.B., Gulamhusein, H., Conrad-Hengerer, I., et al., Efficacy, safety, and risk factors for failure of standalone ab interno gelatin microstent implantation versus standalone trabeculectomy. Ophthalmology. 2017 Nov 1;124(11):1579–88.
19. Sarkisian, S.R., Grover, D.S., Gallardo, M.J., et al., iStent infinite study group. effectiveness and safety of iStent infinite trabecular micro-bypass for uncontrolled glaucoma. Journal of Glaucoma. 2023 Jan 17;32(1):9–18.
20. Katz, L.J., Erb, C., Carceller Guillamet, A., et al., Longterm titrated IOP control with one, two, or three trabecular micro-bypass stents in open-angle glaucoma subjects on topical hypotensive medication: 42-month outcomes. Clinical Ophthalmology. 2018 Jan 31:255–62.
21. Samuelson, T.W., Sarkisian Jr, S.R., Lubeck, D.M., et al., Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology. 2019 Jun 1;126(6):811–21.
22. Samuelson, T.W., Chang, D.F., Marquis, R., et al., A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: the HORIZON study. Ophthalmology. 2019 Jan 1;126(1):29–37.
23. Holmes, D.P., Clement, C.I., Nguyen, V., et al., Comparative study of 2-year outcomes for Hydrus or iStent inject microinvasive glaucoma surgery implants with cataract surgery. Clinical & Experimental Ophthalmology. 2022 Apr;50(3):303–11.
24. Grover, D.S., Godfrey, D.G., Smith, O., et al., Gonioscopyassisted transluminal trabeculotomy, ab interno trabeculotomy: technique report and preliminary results. Ophthalmology. 2014 Apr 1;121(4):855–61.
25. Grover, D.S., Smith, O., Fellman, R.L., et al., Gonioscopyassisted transluminal trabeculotomy: an ab interno circumferential trabeculotomy: 24 months follow-up. Journal of Glaucoma. 2018 May 1;27(5):393–401.
26. Faria, B.M., Costa, V.P., Melillo, G.H., et al., Gonioscopyassisted transluminal trabeculotomy for glaucoma: 1-year outcomes and success predictors. Journal of Glaucoma. 2022 Jun 1;31(6):443–8.
27. Gallardo, M.J., 36-month effectiveness of ab-interno canaloplasty standalone versus combined with cataract surgery for the treatment of open-angle glaucoma. Ophthalmology Glaucoma. 2022 Sep 1;5(5):476–82.
28. Denis, P., Hirneiß, C., Durr, G.M., et al., Two-year outcomes of the MINIject drainage system for uncontrolled glaucoma from the STAR-I first-in-human trial. British Journal of Ophthalmology. 2022 Jan 1;106(1):65–70.
29. Lass, J.H., Benetz, B.A. He, J., et al., Corneal endothelial cell loss and morphometric changes 5 years after phacoemulsification with or without CyPass micro-stent. American Journal of Ophthalmology. 2019 Dec 1;208:211–8.
30. Denis, P., Hirneiß, C., Reddy, K.P., et al., A first-in-human study of the efficacy and safety of MINIject in patients with medically uncontrolled open-angle glaucoma (STAR-I). Ophthalmology Glaucoma. 2019 Sep 1;2(5):290–7.
31. Katz, L.J., Doan L., Applegate, D., Davis, C., et al., Successful reduction in topical medication burden in glaucoma patients at 3 years with iDose intracameral therapy versus timolol. Investigative Ophthalmology & Visual Science. 2022 Jun 1;63(7):3696-A0381.
32. Glaukos’ iDose TR demonstrates sustained IOP reduction and favorable safety profile over 24 months in Phase 2b study [Internet]. investors.glaukos.com [cited 2023 May 30]. Available at: investors.glaukos.com/investors/news/news-details/2021/Glaukos-iDose-TR-Demonstrates-Sustained-IOP-Reductionand-Favorable-Safety-Profile-Over-24-Months-in-Phase-2b-Study/default.aspx [accessed 14 July 2023].
33. An intraocular drug-eluting delivery device for glaucoma [Internet]. Glaucoma Today. Available at: glaucomatoday. com/articles/2020-nov-dec/idose-an-intraocular-drug-elutingdelivery-device-for-glaucoma [accessed 14 July 2023].

Dr Aaron Wong BSc MBChB FRANZCO is an ophthalmologist from Auckland, New Zealand. He has undertaken fellowships in glaucoma in Auckland and general ophthalmology in Melbourne. He is a glaucoma fellow at Sydney Eye Hospital.

Dr Ridia Lim MBBS MPH FRANZCO is a senior cataract and glaucoma surgeon, who is the Head of the Glaucoma Unit at Sydney Eye Hospital and a director at Hunter St Eye Specialists in Parramatta. She is the immediate past Vice Chair of the Australian and New Zealand Glaucoma Society (ANZGS), a senior lecturer at University of Sydney, and is on the Scientific Program Executive Committee for Royal Australian and New Zealand College of Ophthalmology. She is a Board Director of Sight For All and serves on the FGB! registry steering committee.