mistory
Myopia
Insights Through the
Lens of Omics
WRITERS Drs Nina Riddell, Loretta G. Vocale, and Melanie J. Murphy
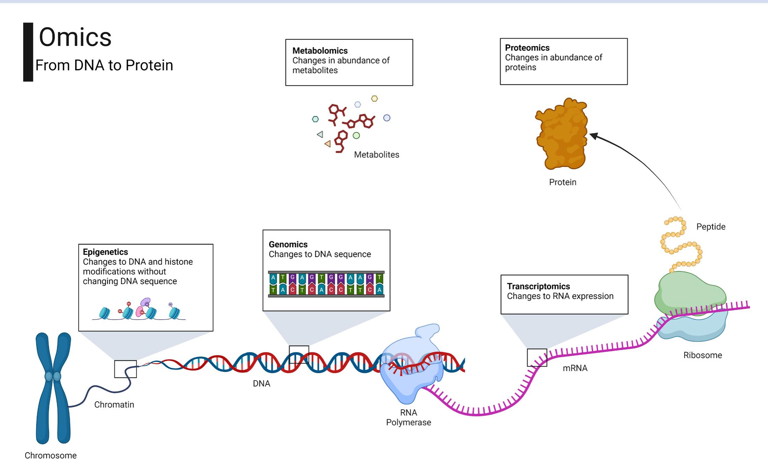
Figure 1: Summary of omics approaches. Omics can be applied to different layers of a biological system such as genomics, transcriptomics, proteomics, epigenomics, and metabolomics. Image created with BioRender.com
Advances in omics techniques, encompassing genome-wide association studies (GWAS), transcriptomics, proteomics, and metabolomics, have revolutionised our understanding of the pathogenesis of complex diseases. This article elucidates the utility of these approaches in dissecting the molecular underpinnings of myopia and its secondary sequalae. The authors argue that ‘omics’ techniques provide the ideal platform for not only understanding the physiological processes underlying myopia, but also a future basis for the development of personalised, precision interventions.
Knowledge that the worldwide prevalence of myopia is dramatically increasing would be of no surprise to those in practice.1-3 Current data estimates the incidence of myopia at between 20–40%, with rates as high as 80% in South East Asia.4-6 Clearly, there is a heritability component underlying predisposition towards developing myopia,7-9 but converging epidemiological and experimental evidence highlights the substantial contribution of environmental factors in driving the condition.7,10
From an epidemiological perspective, we see increases in myopia incidence coinciding with the introduction of universal access to education and higher rates of continued study, as well as factors such as reduced outdoor light exposure and increased close work and technology use.11-15 These observations support the conceptualisation of myopia as a physiological adaptation of a biological system to modern visual demands. Unfortunately, a corollary of greater rates of myopia, and thus high myopia, is the greater population risk of secondary, sightthreatening conditions and blindness.1,16,17 Thus, understanding the mechanisms underlying the abnormal elongation and volume characteristic of the condition is imperative for developing effective, evidencebased, and accessible interventions and therapeutic management strategies, and to relieve the significant socio-economic burden of the condition.18,19
Basic research provides the ideal avenue for achieving this goal. Animal models designed to replicate the induction of refractive error via ocular occlusion or defocussing lenses show a large degree of cross species conservation of both morphological growth and physiological responses, that closely mirror the characteristic features of myopia.20-22 The chick, our model of choice, is a popular animal model23,24 as chicks rapidly and reliably respond to experimental manipulation and exhibit growth changes remarkably like humans on most physical and physiological levels. Other commonly used species are tree shrews, guinea pigs, monkeys, and zebra fish.25
INDUCING REFRACTIVE ERROR
Experimental models generate growth analogous to myopia by exploiting refractive compensation mechanisms and measuring changes during the induction of form deprivation (FD) or blur, or recovery from such.22,23,25-27 Growth following FD induction is not unlike growth associated with visual disease or ocular occlusion that eliminates or degrades the image on the retina in humans.28-32 Comparatively, inducing signed blur with defocussing lenses (LD) drives a more precise refractive compensation process, generating growth adaptation towards the power of the lens. Both techniques cause the eye to rapidly initiate adjustments in axial dimensions to compensate for the loss of focus, measurable within hours.33 Alterations in axial length and vitreous chamber depth are accompanied by alterations in the thickness of the vascular choroid,34-38 decreased fluid flow and changes in vascular dimensions, in conjunction with changes in vascular and lymphatic structure,37,39-41 remodelling of the scleral extracellular matrix, and changes in cell density.42-46 Finally, the gross morphological structure and ultrastructure of the retina is also altered in myopia.39,47-49 This includes damage to cone outer segments, alterations to inner segments, density changes,47 and altered glial function,50-52 with many of these changes indicative of metabolic dysfunction and inflammation.53
The ability to induce refractive error has enabled investigation of the mechanisms driving growth and potential avenues for disrupting such growth, thus ‘preventing’ myopia. Many groups have focussed on pharmacological agents, neurotransmitters, peptides and other molecules,28,49,54-60 as well as temporal, spatio-temporal61-63 and wavelength64-67 properties of light using combinations of animal rearing, immunohistochemical, molecular, and electrophysiological techniques to probe the issue.
While a number of these investigations have reported meaningful findings contributing to our understanding of myopia development, we have not yet been able to find a resolution on the signal driving refractive change, and thus must look more systematically through the powerful lens of ‘omics’ to address the question.
OMICS TECHNIQUES
Biological systems are multi-layered. DNA housed inside our cells is transcribed into RNA, which is then translated into proteins. Proteins serve as the workhorses of the cell, performing a wide array of functions including catalysing metabolic reactions as enzymes, providing structural support, and regulating cellular processes. They interact with metabolites – small molecules involved in metabolism – by facilitating their conversion into energy, signalling molecules, or building blocks for cellular structures. Metabolites, in turn, influence cellular processes and can feedback to affect gene expression and protein activity, creating a dynamic and interconnected network essential for life. Epigenetic modifications such as DNA methylation add another layer of complexity, influencing how genes are expressed in response to environmental factors without altering the DNA sequence itself. Omics techniques allow researchers to examine, at a broad scale, changes within each of these layers – the epigenome, genes, transcripts, proteins, and metabolites – and to dissect the complex interactions of these molecules in health and disease68 (Figure 1).
Genome-wide association studies (GWAS) involve obtaining DNA samples, typically from peripheral blood, and scanning them for hundreds of thousands of genetic markers such as single nucleotide polymorphisms, to identify those associated with specific traits or diseases.69 Human GWAS have linked hundreds of genes to axial length70 and refractive error.9
While these analyses can provide detailed information on the genetic variations that predispose some individuals to myopia, most of the identified loci have small effect sizes, and only a small portion of the variation in spherical equivalent can be explained by genetic variation.9
By comparison, transcriptomics, proteomics, and metabolomics studies directly profile the expression or abundance of molecules within the tissues or biofluids expected to be affected by a disease. Because of this, they can offer insight into how genetic and environmental factors combine to determine the functional state of a tissue. Human ocular tissue and biofluid specimens aren’t easily obtained and, when available, are often affected by disease states in addition to myopia.71,72
Consequently, studies have primarily used animal models to examine how myopia affects the ocular transcriptome, proteome, and metabolome. Such studies have identified thousands of differentially expressed genes and proteins,73 and hundreds of differentially expressed metabolites,71,74 in the retina, choroid, and sclera, in models of occlusion and lens-induced myopia. Collectively, refractive error ‘omics’ studies demonstrate that no single molecule or process controls eye growth; indeed, human and animal studies have implicated genes involved in the development and functioning of every anatomical component of the eye, and all cell types of the neurosensory retina.9,75,76 Although the molecular processes underlying myopia are complex, when the implicated molecules are grouped together systematically, commonalities emerge that facilitate a mechanistic understanding of the processes involved.
LINKING OMICS BETWEEN HUMANS AND ANIMAL MODELS
We systematically compared the genes implicated in human GWAS of refractive error with those differentially expressed in the retina, retinal pigment epithelium (RPE), choroid, and sclera in animal models of lensand occluder-induced myopia. We found that gene and protein expression changes are conserved across animal models of environmentally driven myopia. The genes implicated in these animal models overlap significantly with the genes near human GWAS refractive error loci.73 These results are consistent with a model in which genetic and environmental factors control ocular growth via similar biological pathways, and/ or a model in which genetic variants alter susceptibility to environmental factors rather than being causative in and of themselves, as previously suggested.77
When we looked more closely at the pattern of overlapping findings across studies, we found that, as pointed out in many individual studies,77-79 different genes are involved in the onset versus the persistence and progression of refractive change in animal models. Interestingly, the genes linked to myopia in humans were most like those involved in the early stages of myopia development in animals.
“ … understanding the mechanisms underlying the abnormal elongation and volume characteristics of myopia… is imperative for developing effective, evidence-based, and accessible interventions and therapeutic management strategies ”
Retinal Neurotransmission
Many of the genes implicated in myopia GWAS, and differentially expressed in the retina during early myopia induction in animals, are involved in retinal neurotransmission, consistent with the established understanding that environmentally-induced myopia involves a light-dependent signalling cascade initiated in the retina that ultimately induces scleral remodelling. Here, identification of genes involving phototransduction, ion transport, and glutamate signalling have suggested a key role for the photoreceptor-bipolar cell interface.9,33,75,80-85
Electroretinogram studies have also identified changes to photoreceptor function and the balance of ON/OFF bipolar cell signalling in human myopes86-88 and animal lensand occlusion-myopia models.33,89 GABA, an essential neurotransmitter in shaping signal transmission from bipolar cells to other neurons in the retina, has also been implicated in lensand occluderinduced myopia in animals.85,90 The primary GABAergic channels implicated in experimental myopia in animals are ionotropic, which implicates ion homeostasis, particularly chloride91,92 and associated fluid movement.75,84,93,94
“ Evidence to date has shown that effective interventions can target the myopic homeostasis shift at different levels ”
Energy Metabolism
The functional activity and metabolic needs of the retina are closely related. It is one of the body’s most energy-demanding tissues, with most of this energy being used to power photoreceptor Na+/K+ ATPase ion channels.95,96 Energy metabolism also provides the biosynthetic precursors required for structural remodelling and growth of tissues.97 The retina requires both glycolysis and oxidative phosphorylation for vision,95 and omics studies of myopia have repeatedly identified extensive changes in the abundance of molecules in the retina participating in both of these metabolic processes.33,80,84,90,98-103 Dysregulation of energy metabolism in the sclera has also been demonstrated, where hypoxia-induced changes within the glycolytic pathway during myopia induction have recently been shown to increase lactate levels, ultimately promoting scleral extracellular matrix remodelling and thinning via lactate-derived histone lactylation (a newly identified form of epigenetic modification),104 and contributing to an advancing knowledge about the epigenetic changes associated with myopia development.105,106 Although human studies cannot directly profile the metabolic state of retina and sclera, human GWAS have linked glucose metabolism with axial length and myopia age of onset,8, 83,107 and studies of serum samples from patients with high myopia have identified metabolic profiles indicative of abnormal glycometabolism.108
Immune Responses and Inflammation
Immune and inflammatory processes have long been known to promote structural remodelling of tissues,109,110 and these processes have been increasingly implicated in the myopic eye.111 Here, omics studies suggest a particular role for the complement system, a key component of innate immunity that also assists in maintaining metabolic homeostasis in cells.84,112-114 Indeed, when we meta-analysed datasets profiling gene expression in the retina, RPE, and choroid during refractive error induction in the widely used chick animal model, many of the growth-specific genes (i.e., genes showing bidirectional expression across myopia and hyperopia conditions) encoded proteins that form a highly connected network involved in inflammation-mediated extracellular matrix remodelling.101 This finding was recently mirrored in a proteomic study of the cornea in human myopes.115 Mechanistically, in addition to directly driving structural change, prolonged subclinical inflammation in myopia may cause endothelial dysfunction, reducing oxygen supply, and exacerbating hypoxia-linked scleral remodelling.111
CONCLUSION
The functional, metabolic and inflammatory processes that we’ve described are part of a large-scale shift in ocular homeostasis that occurs during myopia development. Outside of the ‘omics’ literature, targeted studies have identified changes in the abundance of many single molecules such as dopamine, VIP, ERG1, ZENK, BMP (and more).5,25,49,116-119 And within the omics space, processes not described in depth here, such as circadian rhythm disturbance,9 have also been documented across studies. This shift in homeostasis provides a cascade of signals propagating from the retina through to the sclera that induce changes in the expression of genes involved in regulating cellular and extracellular structure,73 ultimately resulting in axial elongation of the eye. This abnormal eye growth, in turn, has long been understood to produce mechanical and ischemic/vascular stress that underlies the development of secondary pathologies.10,16,120-123 Many of the biological pathways associated with myopia’s secondaries – such as oxidative stress and complement-mediated inflammation – are implicated in animal models of myopia before the emergence of any pathological structural change,80,101 suggesting that environmentally-induced expression shifts act together with structural changes to promote the development of age-related secondary complications in myopic eyes.
Just as the molecular mechanisms underlying myopia and its sequelae are complex, it is likely that the mechanisms behind the effectiveness of myopia-control interventions are complex as well. Evidence to date has shown that effective interventions can target the myopic homeostasis shift at different levels. For example, behavioural interventions, like spending greater time outdoors, presumably target the maladaptive neurotransmission patterns underlying the retina-sclera signalling myopia. Thus, while current treatments have a broad spectrum approach showing a degree of efficacy, an ‘omics’ perspective may provide greater nuance in elaborating the cellular underpinnings from a systems biology approach, providing potential for precise and individualised care.
Dr Nina Riddell BPsySci (Hon), PhD is a lecturer in neuroscience at the Department of Psychology and Counselling at La Trobe University. Her PhD, completed at La Trobe University, focussed on the systems biology of myopia and associated secondary conditions. She has held research contracts with Hoya Vision.

Dr Loretta Vocale BMedSci (Hon), PhD is a lecturer in neuroscience and psychology at RMIT University. Her PhD investigated the biological mechanisms underlying myopia. She is completing an ARCfunded post-doctorate research fellowship focussed on understanding how brain inflammation can be detected by observing changes in the retina.

Dr Melanie Murphy BBehavSci (Hon), PhD is a lecturer in the Department of Psychology and Counselling at La Trobe University. Her expertise is in vision neuroscience ranging from the cellular basis of visual processing in animal and human models, to cognitive development and neurodegeneration of visual processing.

References available at mivision.com.au